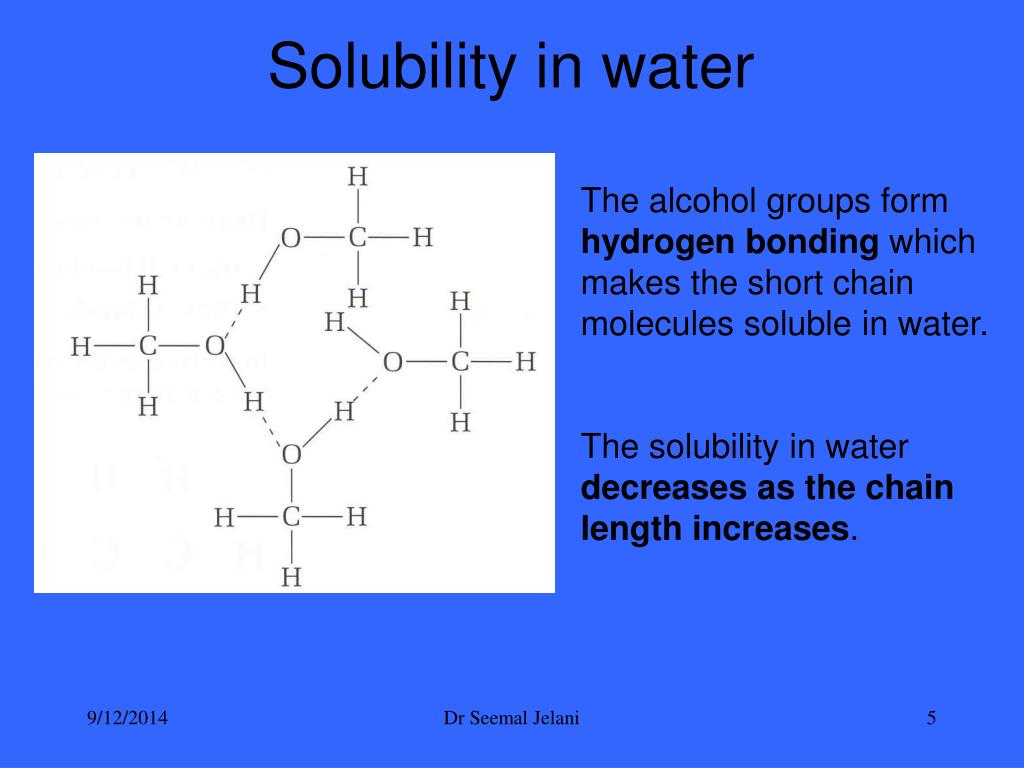
Is Ethyl Alcohol Soluble In Water . Mixing the two in any proportion generates a single solution. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Small alcohols are completely soluble in water; Small alcohols are completely soluble in water; Alcohols can be considered derivatives of. We find that diethyl ether is much less soluble in water. Alcohols are polar and have considerable water solubility. Solubility of alcohols in water. The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. We find that diethyl ether is much less soluble in water. Is it capable of forming hydrogen bonds with water? Is it capable of forming hydrogen bonds with water? Mixing the two in any proportion generates a single solution.
from www.slideserve.com
Small alcohols are completely soluble in water; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Mixing the two in any proportion generates a single solution. Alcohols are polar and have considerable water solubility. Alcohols can be considered derivatives of. We find that diethyl ether is much less soluble in water. Solubility of alcohols in water. Small alcohols are completely soluble in water; Is it capable of forming hydrogen bonds with water? Is it capable of forming hydrogen bonds with water?
PPT ALCOHOL PowerPoint Presentation, free download ID4296802
Is Ethyl Alcohol Soluble In Water The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Small alcohols are completely soluble in water; We find that diethyl ether is much less soluble in water. Is it capable of forming hydrogen bonds with water? Alcohols are polar and have considerable water solubility. Mixing the two in any proportion generates a single solution. We find that diethyl ether is much less soluble in water. Is it capable of forming hydrogen bonds with water? Mixing the two in any proportion generates a single solution. Small alcohols are completely soluble in water; Solubility of alcohols in water. The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Alcohols can be considered derivatives of.
From www.semanticscholar.org
Table 2 from Solubility of NaCl, NaBr, and KCl in Water, Methanol Is Ethyl Alcohol Soluble In Water Small alcohols are completely soluble in water; We find that diethyl ether is much less soluble in water. Is it capable of forming hydrogen bonds with water? Is it capable of forming hydrogen bonds with water? The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Alcohols are polar and have considerable water. Is Ethyl Alcohol Soluble In Water.
From www.scribd.com
B. Solubility Test Methods Samples Hexane Water Ethyl Alcohol 6N Hcl Is Ethyl Alcohol Soluble In Water We find that diethyl ether is much less soluble in water. Small alcohols are completely soluble in water; The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Solubility of alcohols in water. Alcohols are polar and have considerable water solubility. Alcohols can be considered derivatives of. Mixing the two in any proportion. Is Ethyl Alcohol Soluble In Water.
From www.semanticscholar.org
[PDF] Solubility, liquidliquid equilibrium and critical states for the Is Ethyl Alcohol Soluble In Water Solubility of alcohols in water. The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Is it capable of forming hydrogen bonds with water? Alcohols are polar and have considerable water solubility. We find that diethyl ether is much less soluble in water. Small alcohols are completely soluble in water; Explain why alcohols. Is Ethyl Alcohol Soluble In Water.
From anneliesevanderpolnetworth.blogspot.com
draw the structure of ethanol molecule Is Ethyl Alcohol Soluble In Water Is it capable of forming hydrogen bonds with water? We find that diethyl ether is much less soluble in water. Mixing the two in any proportion generates a single solution. Is it capable of forming hydrogen bonds with water? Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble.. Is Ethyl Alcohol Soluble In Water.
From www.slideserve.com
PPT Mixing ethanol and water lab… PowerPoint Presentation, free Is Ethyl Alcohol Soluble In Water Mixing the two in any proportion generates a single solution. Solubility of alcohols in water. Alcohols can be considered derivatives of. Small alcohols are completely soluble in water; We find that diethyl ether is much less soluble in water. Mixing the two in any proportion generates a single solution. Alcohols are polar and have considerable water solubility. Is it capable. Is Ethyl Alcohol Soluble In Water.
From courses.lumenlearning.com
14.3 Physical Properties of Alcohols The Basics of General, Organic Is Ethyl Alcohol Soluble In Water We find that diethyl ether is much less soluble in water. Is it capable of forming hydrogen bonds with water? Solubility of alcohols in water. Is it capable of forming hydrogen bonds with water? Mixing the two in any proportion generates a single solution. The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water. Is Ethyl Alcohol Soluble In Water.
From www.sciencephoto.com
Water and ethanol Stock Image C044/8783 Science Photo Library Is Ethyl Alcohol Soluble In Water Mixing the two in any proportion generates a single solution. Small alcohols are completely soluble in water; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Alcohols are polar and have considerable water solubility. Is it capable of forming hydrogen bonds with water? The second key implication of. Is Ethyl Alcohol Soluble In Water.
From www.youtube.com
Is C2H5OH (Ethanol) Soluble or Insoluble in Water? YouTube Is Ethyl Alcohol Soluble In Water We find that diethyl ether is much less soluble in water. Is it capable of forming hydrogen bonds with water? Is it capable of forming hydrogen bonds with water? We find that diethyl ether is much less soluble in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not. Is Ethyl Alcohol Soluble In Water.
From www.researchgate.net
Solubility of Selected Compounds in Water and Ethanol Download Table Is Ethyl Alcohol Soluble In Water Small alcohols are completely soluble in water; Is it capable of forming hydrogen bonds with water? Is it capable of forming hydrogen bonds with water? Mixing the two in any proportion generates a single solution. We find that diethyl ether is much less soluble in water. Small alcohols are completely soluble in water; The second key implication of hydrogen bonding. Is Ethyl Alcohol Soluble In Water.
From askfilo.com
3. Fractional distillation of ethyl alcoholwater mixture gives a mixture.. Is Ethyl Alcohol Soluble In Water The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Mixing the two in any proportion generates a single solution. Mixing the two in any proportion generates a single solution. Small alcohols are completely soluble in water; Alcohols are polar and have considerable water solubility. Small alcohols are completely soluble in water; Explain. Is Ethyl Alcohol Soluble In Water.
From www.slideserve.com
PPT Part I Solubility, Factors Affecting Solubility PowerPoint Is Ethyl Alcohol Soluble In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Small alcohols are completely soluble in water; Is it capable of forming hydrogen bonds with water? We find that diethyl ether is. Is Ethyl Alcohol Soluble In Water.
From www.youtube.com
4 2 4 alcohols solubility and solvent polarity YouTube Is Ethyl Alcohol Soluble In Water Is it capable of forming hydrogen bonds with water? Small alcohols are completely soluble in water; Mixing the two in any proportion generates a single solution. Is it capable of forming hydrogen bonds with water? Mixing the two in any proportion generates a single solution. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water. Is Ethyl Alcohol Soluble In Water.
From www.youtube.com
C2H5OH + H2O (Ethanol + Water) YouTube Is Ethyl Alcohol Soluble In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Small alcohols are completely soluble in water; Alcohols are polar and have considerable water solubility. Mixing the two in any proportion generates a single solution. Solubility of alcohols in water. Is it capable of forming hydrogen bonds with water?. Is Ethyl Alcohol Soluble In Water.
From slidetodoc.com
Organic Chemistry 5 th Edition L G Wade Is Ethyl Alcohol Soluble In Water Solubility of alcohols in water. Alcohols are polar and have considerable water solubility. Small alcohols are completely soluble in water; Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Small alcohols are completely soluble in water; Is it capable of forming hydrogen bonds with water? We find that. Is Ethyl Alcohol Soluble In Water.
From byjus.com
Among primary, secondary and tertiary, which alcohol is more soluble in Is Ethyl Alcohol Soluble In Water We find that diethyl ether is much less soluble in water. Alcohols are polar and have considerable water solubility. Mixing the two in any proportion generates a single solution. Is it capable of forming hydrogen bonds with water? Small alcohols are completely soluble in water; Solubility of alcohols in water. Alcohols can be considered derivatives of. Explain why alcohols and. Is Ethyl Alcohol Soluble In Water.
From mavink.com
Ethanol Density Chart Is Ethyl Alcohol Soluble In Water Small alcohols are completely soluble in water; The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. We find that diethyl ether is much less soluble in water. Alcohols are polar and. Is Ethyl Alcohol Soluble In Water.
From mungfali.com
Solubility Rules Flowchart Chart Chemistry Is Ethyl Alcohol Soluble In Water Mixing the two in any proportion generates a single solution. We find that diethyl ether is much less soluble in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Small alcohols are completely soluble in water; Is it capable of forming hydrogen bonds with water? Alcohols can. Is Ethyl Alcohol Soluble In Water.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6009487 Is Ethyl Alcohol Soluble In Water We find that diethyl ether is much less soluble in water. Small alcohols are completely soluble in water; Solubility of alcohols in water. Small alcohols are completely soluble in water; Mixing the two in any proportion generates a single solution. Is it capable of forming hydrogen bonds with water? Explain why alcohols and ethers of four or fewer carbon atoms. Is Ethyl Alcohol Soluble In Water.
From www.numerade.com
SOLVEDWhy are some molecular solids (such as sugar or ethyl alcohol Is Ethyl Alcohol Soluble In Water The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Is it capable of forming hydrogen bonds with water? Solubility of alcohols in water. Alcohols can be considered derivatives of. We find that diethyl ether is much less soluble in water. Explain why alcohols and ethers of four or fewer carbon atoms are. Is Ethyl Alcohol Soluble In Water.
From www.researchgate.net
Vapourliquid equilibrium of ethanolwater showing distillation steps Is Ethyl Alcohol Soluble In Water Mixing the two in any proportion generates a single solution. Solubility of alcohols in water. Alcohols are polar and have considerable water solubility. We find that diethyl ether is much less soluble in water. Small alcohols are completely soluble in water; Is it capable of forming hydrogen bonds with water? Explain why alcohols and ethers of four or fewer carbon. Is Ethyl Alcohol Soluble In Water.
From www.numerade.com
Part C Miscible or immiscible pairs? Water and ethyl alcohol Water and Is Ethyl Alcohol Soluble In Water Is it capable of forming hydrogen bonds with water? Alcohols are polar and have considerable water solubility. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Mixing the two in any proportion generates a single solution. Mixing the two in any proportion generates a single solution. Is it. Is Ethyl Alcohol Soluble In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Is Ethyl Alcohol Soluble In Water The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Solubility of alcohols in water. Mixing the two in any proportion generates a single solution. We find that diethyl ether is much less soluble in water. Small alcohols are completely soluble in water; Explain why alcohols and ethers of four or fewer carbon. Is Ethyl Alcohol Soluble In Water.
From www.chegg.com
Solved 1. The solubility of some simple alcohols in water is Is Ethyl Alcohol Soluble In Water Solubility of alcohols in water. Is it capable of forming hydrogen bonds with water? Small alcohols are completely soluble in water; Alcohols can be considered derivatives of. Is it capable of forming hydrogen bonds with water? Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Alcohols are polar. Is Ethyl Alcohol Soluble In Water.
From www.semanticscholar.org
Table 1 from Solubility of NaCl, NaBr, and KCl in Water, Methanol Is Ethyl Alcohol Soluble In Water Alcohols are polar and have considerable water solubility. Small alcohols are completely soluble in water; Alcohols can be considered derivatives of. We find that diethyl ether is much less soluble in water. Is it capable of forming hydrogen bonds with water? Is it capable of forming hydrogen bonds with water? Small alcohols are completely soluble in water; We find that. Is Ethyl Alcohol Soluble In Water.
From radio.egerton.ac.ke
PDF] Solubility Of NaCl, NaBr, And KCl In Water, Methanol,, 53 OFF Is Ethyl Alcohol Soluble In Water Small alcohols are completely soluble in water; We find that diethyl ether is much less soluble in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. We find that diethyl ether is much less soluble in water. Solubility of alcohols in water. Is it capable of forming. Is Ethyl Alcohol Soluble In Water.
From slidetodoc.com
Chapter 13 Alcohols Phenols and Thiols 13 3 Is Ethyl Alcohol Soluble In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. We find that diethyl ether is much less soluble in water. Mixing the two in any proportion generates a single solution. Alcohols are polar and have considerable water solubility. Is it capable of forming hydrogen bonds with water? Solubility. Is Ethyl Alcohol Soluble In Water.
From www.researchgate.net
Triolein solubility in ethanolwater binary mixtures. Expanded view of Is Ethyl Alcohol Soluble In Water We find that diethyl ether is much less soluble in water. Mixing the two in any proportion generates a single solution. Alcohols can be considered derivatives of. The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. Small alcohols are completely soluble in water; We find that diethyl ether is much less soluble. Is Ethyl Alcohol Soluble In Water.
From www.researchgate.net
1. Specific heat of ethyl alcoholwater Download Scientific Diagram Is Ethyl Alcohol Soluble In Water We find that diethyl ether is much less soluble in water. Small alcohols are completely soluble in water; Small alcohols are completely soluble in water; Mixing the two in any proportion generates a single solution. Alcohols are polar and have considerable water solubility. We find that diethyl ether is much less soluble in water. Is it capable of forming hydrogen. Is Ethyl Alcohol Soluble In Water.
From www.researchgate.net
Solubility mole fraction in ethanolwater (δ1 = 26.5147.97 MPa1/2 Is Ethyl Alcohol Soluble In Water Solubility of alcohols in water. The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water solubility. We find that diethyl ether is much less soluble in water. Mixing the two in any proportion generates a single solution. Small alcohols are completely soluble in water; We find that diethyl ether is much less soluble in. Is Ethyl Alcohol Soluble In Water.
From www.researchgate.net
Details of solubility data in waterethanol mixtures, number of data Is Ethyl Alcohol Soluble In Water Is it capable of forming hydrogen bonds with water? Is it capable of forming hydrogen bonds with water? Alcohols are polar and have considerable water solubility. Alcohols can be considered derivatives of. Mixing the two in any proportion generates a single solution. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes. Is Ethyl Alcohol Soluble In Water.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6540865 Is Ethyl Alcohol Soluble In Water Is it capable of forming hydrogen bonds with water? Small alcohols are completely soluble in water; Alcohols are polar and have considerable water solubility. Is it capable of forming hydrogen bonds with water? We find that diethyl ether is much less soluble in water. Alcohols can be considered derivatives of. Small alcohols are completely soluble in water; The second key. Is Ethyl Alcohol Soluble In Water.
From www.semanticscholar.org
Table 1 from Solubility of NaCl, NaBr, and KCl in Water, Methanol Is Ethyl Alcohol Soluble In Water Mixing the two in any proportion generates a single solution. Small alcohols are completely soluble in water; We find that diethyl ether is much less soluble in water. Small alcohols are completely soluble in water; Is it capable of forming hydrogen bonds with water? The second key implication of hydrogen bonding is that the hydroxyl group imparts much greater water. Is Ethyl Alcohol Soluble In Water.
From www.slideserve.com
PPT ALCOHOL PowerPoint Presentation, free download ID4296802 Is Ethyl Alcohol Soluble In Water Solubility of alcohols in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Mixing the two in any proportion generates a single solution. Small alcohols are completely soluble in water; Is it capable of forming hydrogen bonds with water? We find that diethyl ether is much less. Is Ethyl Alcohol Soluble In Water.
From slideplayer.com
Chemeketa Community College ppt download Is Ethyl Alcohol Soluble In Water Small alcohols are completely soluble in water; Mixing the two in any proportion generates a single solution. Is it capable of forming hydrogen bonds with water? Is it capable of forming hydrogen bonds with water? Alcohols are polar and have considerable water solubility. We find that diethyl ether is much less soluble in water. Small alcohols are completely soluble in. Is Ethyl Alcohol Soluble In Water.
From rayb78.github.io
Solubility In Water Chart Is Ethyl Alcohol Soluble In Water We find that diethyl ether is much less soluble in water. Mixing the two in any proportion generates a single solution. Is it capable of forming hydrogen bonds with water? Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Small alcohols are completely soluble in water; Solubility of. Is Ethyl Alcohol Soluble In Water.