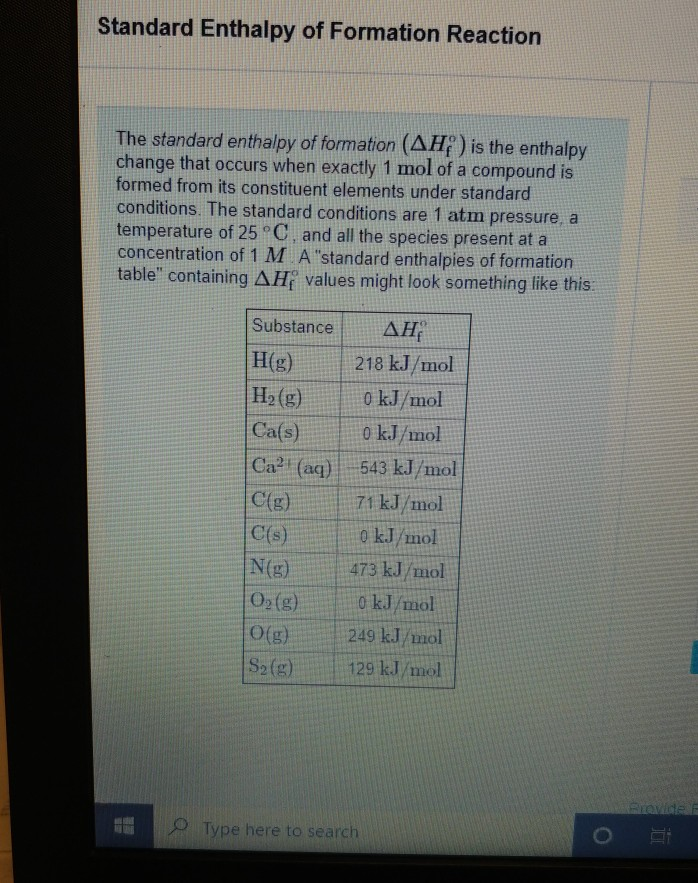
Standard Enthalpy Of Formation Rxn . A formation equation is written. The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. The nought superscript means standard state. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states.
from www.chegg.com
Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. A formation equation is written. The nought superscript means standard state. The standard enthalpy (heat) of reaction is given by δh o rxn.
Solved Standard Enthalpy of Formation Reaction The standard
Standard Enthalpy Of Formation Rxn A formation equation is written. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy (heat) of reaction is given by δh o rxn. The nought superscript means standard state. A formation equation is written. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Rxn The standard enthalpy (heat) of reaction is given by δh o rxn. The nought superscript means standard state. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. Consider the general reaction \[ aa + bb \rightarrow. Standard Enthalpy Of Formation Rxn.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Rxn Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] The nought superscript means standard state. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. Tabulated values of standard enthalpies of formation can. Standard Enthalpy Of Formation Rxn.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Rxn The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. A formation equation is written. Tabulated values of standard. Standard Enthalpy Of Formation Rxn.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy (heat) of reaction is given by δh o rxn. Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] The nought superscript means standard state. The standard. Standard Enthalpy Of Formation Rxn.
From www.chegg.com
Solved Standard Enthalpy of Formation Reaction The standard Standard Enthalpy Of Formation Rxn The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The nought superscript means standard state. The standard enthalpy (heat) of reaction is given by δh o rxn. A formation equation is written. The standard enthalpy of formation (δh of) is the heat change that results. Standard Enthalpy Of Formation Rxn.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of reaction (δh rxn) is the. Standard Enthalpy Of Formation Rxn.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation Rxn The nought superscript means standard state. Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The. Standard Enthalpy Of Formation Rxn.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of formation. Standard Enthalpy Of Formation Rxn.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Rxn The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy (heat) of reaction is given by δh o rxn.. Standard Enthalpy Of Formation Rxn.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Rxn Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs. Standard Enthalpy Of Formation Rxn.
From www.youtube.com
6.6 Standard Enthalpy of Formation and Reaction YouTube Standard Enthalpy Of Formation Rxn The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy (heat) of reaction is given by δh o rxn.. Standard Enthalpy Of Formation Rxn.
From slideplayer.com
General Chemistry CHEM 101 (3+1+0). ppt download Standard Enthalpy Of Formation Rxn A formation equation is written. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change. Standard Enthalpy Of Formation Rxn.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Rxn Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a. Standard Enthalpy Of Formation Rxn.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Rxn The standard enthalpy (heat) of reaction is given by δh o rxn. Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] A formation equation is written. The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The nought. Standard Enthalpy Of Formation Rxn.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib Standard Enthalpy Of Formation Rxn Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of formation is a measure. Standard Enthalpy Of Formation Rxn.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose. Standard Enthalpy Of Formation Rxn.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Rxn The nought superscript means standard state. A formation equation is written. The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed. Standard Enthalpy Of Formation Rxn.
From slideplayer.com
General Chemistry CHEM 101 (3+1+0). ppt download Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation Rxn.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The nought superscript means standard state. The standard enthalpy (heat) of reaction is given by δh o rxn. A formation equation is written. The standard enthalpy of formation (δh of) is the. Standard Enthalpy Of Formation Rxn.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1.. Standard Enthalpy Of Formation Rxn.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Rxn The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs. Standard Enthalpy Of Formation Rxn.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Rxn The nought superscript means standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. Consider the general reaction \[ aa. Standard Enthalpy Of Formation Rxn.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Rxn A formation equation is written. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states.. Standard Enthalpy Of Formation Rxn.
From people.chem.umass.edu
to Adobe GoLive 6 Standard Enthalpy Of Formation Rxn A formation equation is written. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with. Standard Enthalpy Of Formation Rxn.
From studyafrikander.z13.web.core.windows.net
Calculate Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Rxn The standard enthalpy (heat) of reaction is given by δh o rxn. Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. The standard. Standard Enthalpy Of Formation Rxn.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Rxn Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. The standard enthalpy. Standard Enthalpy Of Formation Rxn.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. A formation equation is written. The standard enthalpy. Standard Enthalpy Of Formation Rxn.
From www.chemistrylearner.com
Heat (Enthalpy) of Reaction Definition, Examples, & Formula Standard Enthalpy Of Formation Rxn The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The nought superscript. Standard Enthalpy Of Formation Rxn.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. Consider the general reaction \[ aa + bb. Standard Enthalpy Of Formation Rxn.
From www.numerade.com
SOLVED Calculate ΔH∘rxn for this reaction using standard enthalpies Standard Enthalpy Of Formation Rxn The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of formation (δh of) is the heat change that. Standard Enthalpy Of Formation Rxn.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Rxn The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard states. The nought superscript means standard state. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard. Standard Enthalpy Of Formation Rxn.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Rxn Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] The nought superscript means standard state. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. The standard enthalpy of formation is a measure. Standard Enthalpy Of Formation Rxn.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Rxn Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. Consider the general reaction \[ aa + bb \rightarrow cc + dd \label{7.8.3}\] The nought superscript means standard state. A formation equation is written. The standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation Rxn.
From www.numerade.com
SOLVED Calculate the standard Gibbs energy of the reaction CO(g Standard Enthalpy Of Formation Rxn A formation equation is written. The nought superscript means standard state. The standard enthalpy of formation (δh of) is the heat change that results when one mole of a compound is formed from its elements (in most stable form/natural) at a pressure of 1. The standard enthalpy of reaction (δh rxn) is the enthalpy change that occurs when a reaction. Standard Enthalpy Of Formation Rxn.
From slideplayer.com
Thermochemistry Chapter ppt download Standard Enthalpy Of Formation Rxn The standard enthalpy (heat) of reaction is given by δh o rxn. Tabulated values of standard enthalpies of formation can be used to calculate enthalpy changes for any reaction involving substances whose \(\delta{h_f^o}\) values are known. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products in. Standard Enthalpy Of Formation Rxn.