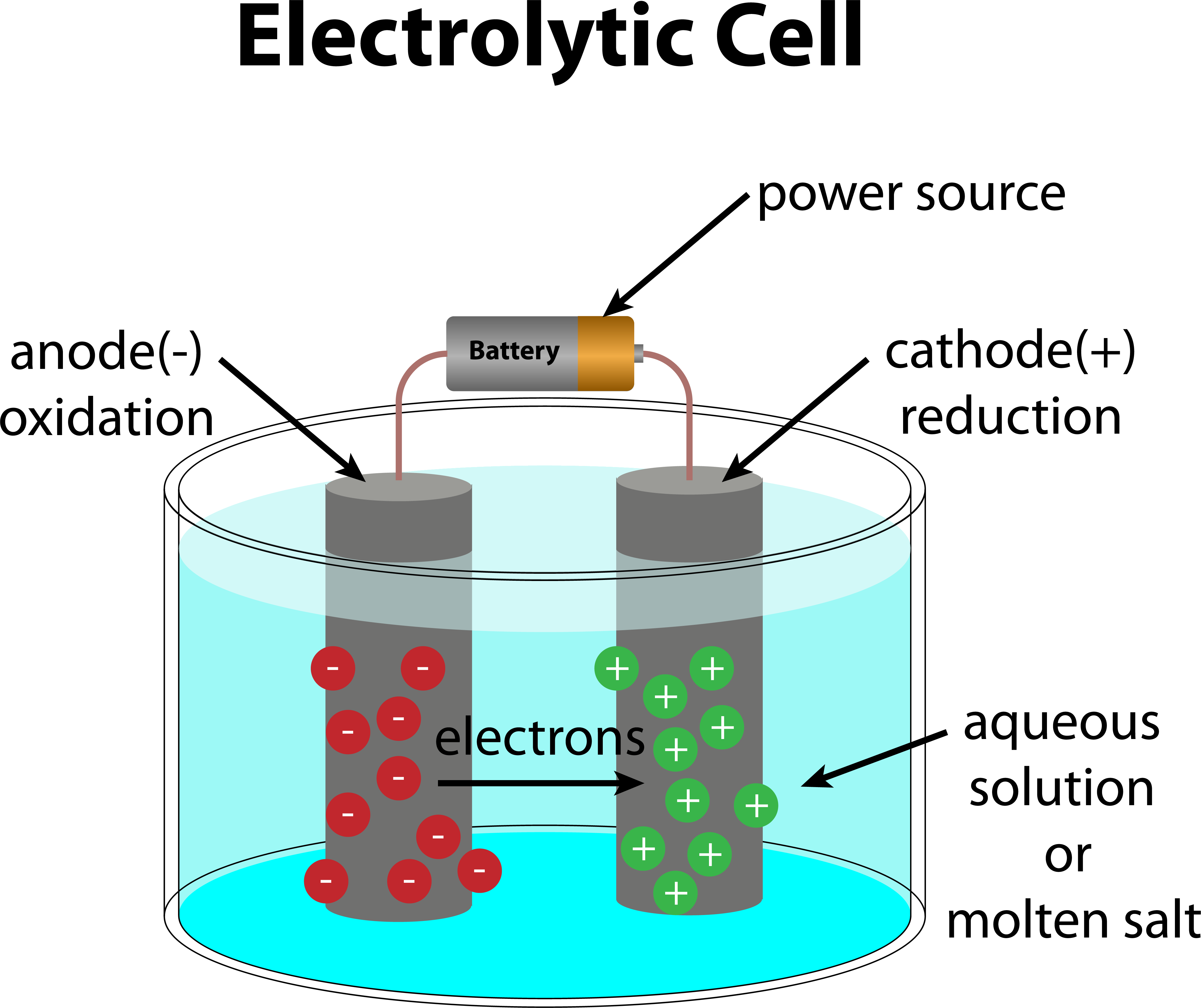
Difference Between Electrochemical Cell And Electrolytic Cell Class 12 . — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — what is an electrochemical cell? — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to.
from www.yaclass.in
— in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — what is an electrochemical cell? — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge.
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science
Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — what is an electrochemical cell? — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — the most significant difference between an electrolytic cell and an electrochemical cell is that an. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it
From www.youtube.com
Electrochemical Cell vs Electrolytic Cell Electrochemistry YouTube Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — what is an electrochemical cell? — the most significant difference between an electrolytic cell and an electrochemical cell is that an.. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From askfilo.com
These are of two types Electrochemical cell and Electrolytic cell. Sr. N.. Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — what is an electrochemical cell? An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it,. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From learningschoolletopisaln.z22.web.core.windows.net
Types Of Electrochemical Cells Pdf Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — in order to understand the difference between. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — in an electrochemical cell, half cells are placed. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — what is an electrochemical cell? — in. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.pinterest.com
Difference Between Electrochemical Cell and Electrolytic Cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — in an electrochemical cell, half cells are. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.chemca.in
Types of Electrochemical Cells Difference between galvanic cell and Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — the main difference between an electrochemical cell and. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — what is an electrochemical cell? — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — the main difference between an electrochemical cell and an electrolytic cell lies in their. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
Class 12 How electricity is generated in an electrochemical cell YouTube Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. An electrochemical cell is a. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From wiredataheathowiu8.z22.web.core.windows.net
Cathode Charge In Electrolytic Cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — the most significant difference between an electrolytic cell. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From lessonschoolallodial.z13.web.core.windows.net
Electrochemical Cells In Chemistry Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — what is an. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
9.2 Comparison of electrochemical cells (SL) YouTube Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — in an electrochemical cell, half cells. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
Difference Between Galvanic Cell And Electrolytic Cell Electrochemistry Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — in order to understand the. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
Trick to learn difference between electrochemical cell and electrolytic Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — what is an electrochemical cell? — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — the major distinction between an electrochemical cell and an electrolytic cell is that an. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. An electrochemical cell. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From aryan-blogduke.blogspot.com
Daniell Cell Class 12 Difference Between Electrochemical Cell And Electrolytic Cell Class 12 An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — in an electrochemical cell, half cells. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
DIFFERENCES BETWEEN ELECTROLYTIC CELL AND ELECTROCHEMICAL CELL PART1 Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — in order to. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. An electrochemical cell is a. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From circuitlistjames.z21.web.core.windows.net
Diagram Of A Simple Electrolytic Cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — what is an electrochemical cell? — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — the main difference between an electrochemical cell and an electrolytic cell lies in. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — what is an electrochemical cell? An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it —. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.askdifference.com
Electrochemical Cell vs. Electrolytic Cell — What’s the Difference? Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the most significant difference between an electrolytic cell and an electrochemical cell is that an. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — when an electric current is passed through the cell,. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From quizzzonediane.z21.web.core.windows.net
Examples Of Electrochemical Cells Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — what is an electrochemical cell? — the main difference between an electrochemical cell and an electrolytic cell lies in their. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From guidelisteickhoff.z21.web.core.windows.net
Electrochemistry Cell Diagram Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — what is an electrochemical cell? — the most significant difference between an electrolytic cell and an electrochemical cell is. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From knowdifferences.com
Difference between Electrochemical Cell and Electrolytic Cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — what is an electrochemical cell? — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. —. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.artofit.org
Electrochemical cell Artofit Difference Between Electrochemical Cell And Electrolytic Cell Class 12 An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring in it, or use the electrical energy supplied to it to facilitate chemical reactions in it — what is an electrochemical cell? — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — in. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — the. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — what. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From knowdifferences.com
Difference between Electrochemical Cell and Electrolytic Cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — what is an electrochemical cell? — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — the most significant difference between an electrolytic cell and an electrochemical cell is. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
Difference Between Electrolytic Cell and Voltaic Cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — what is an electrochemical cell? — the major distinction between an electrochemical cell and an electrolytic cell is that an. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
3.12Difference between Electrochemical and Electrolytic cell, class Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — what is an electrochemical cell? — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — when an electric current is passed through the cell, ions migrate towards. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From exoyksicy.blob.core.windows.net
Distinguish Between Galvanic Cell And Electrolytic Cell at John Rowe blog Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — the main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — in order to understand the. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — in an electrochemical cell, half cells are placed in different containers and connected via a salt bridge. — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. —. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From www.youtube.com
Comparison between the electrolytic cell and chemical cell YouTube Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — what is an electrochemical cell? — in order to understand the difference between an electrochemical cell and an electrolytic cell, it is important to. — the most significant difference between an electrolytic cell and an electrochemical cell is that an. An electrochemical cell is a device that can generate electrical energy from the chemical reactions occurring. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.
From knowdifferences.com
Difference between Electrochemical Cell and Electrolytic Cell Difference Between Electrochemical Cell And Electrolytic Cell Class 12 — when an electric current is passed through the cell, ions migrate towards the electrodes, where they. — the most significant difference between an electrolytic cell and an electrochemical cell is that an. — the major distinction between an electrochemical cell and an electrolytic cell is that an electrochemical cell does. — in order to understand. Difference Between Electrochemical Cell And Electrolytic Cell Class 12.