Science Geek Molarity . molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. Masses of solute must first be. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. molarity describes the relationship between moles of a solute and the volume of a solution. Click the start quiz button to proceed. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. What is the molarity of. [1] to calculate molarity, you can start. molarity describes the concentration of a solution in moles of solute divided by liters of solution. the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a.
from chemistry.stackexchange.com
Click the start quiz button to proceed. the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. molarity describes the concentration of a solution in moles of solute divided by liters of solution. molarity describes the relationship between moles of a solute and the volume of a solution. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. Masses of solute must first be. What is the molarity of. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. [1] to calculate molarity, you can start. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution.
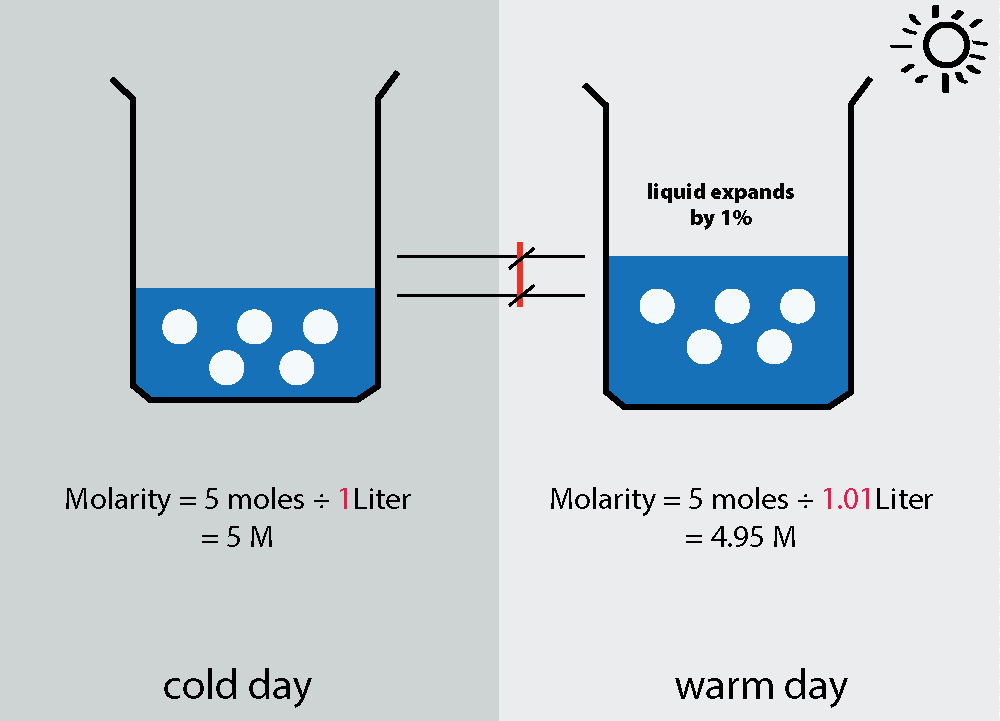
physical chemistry Temperature dependence of molarity and molality
Science Geek Molarity the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. Click the start quiz button to proceed. molarity describes the concentration of a solution in moles of solute divided by liters of solution. [1] to calculate molarity, you can start. Masses of solute must first be. molarity describes the relationship between moles of a solute and the volume of a solution. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. What is the molarity of.
From www.thoughtco.com
Learn How to Calculate Molarity of a Solution Science Geek Molarity What is the molarity of. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. molarity describes the concentration of a solution in moles of solute divided by liters of solution. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. Masses of solute must. Science Geek Molarity.
From www.wikihow.com
4 Ways to Calculate Molarity wikiHow Science Geek Molarity the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. What is the molarity of. molarity the most common unit of concentration is molarity, which. Science Geek Molarity.
From www.youtube.com
Basic molarity calculations YouTube Science Geek Molarity the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. Click the start quiz button to proceed. What is the molarity of. molarity describes the concentration of a solution in moles of solute divided by liters of solution. molar concentration. Science Geek Molarity.
From www.slideserve.com
PPT Molarity PowerPoint Presentation, free download ID2674241 Science Geek Molarity [1] to calculate molarity, you can start. molarity describes the concentration of a solution in moles of solute divided by liters of solution. Masses of solute must first be. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. molarity describes the relationship between moles of a solute and the. Science Geek Molarity.
From www.showme.com
Concentration and Molarity Science, molarity, AP Chemistry ShowMe Science Geek Molarity Masses of solute must first be. Click the start quiz button to proceed. What is the molarity of. [1] to calculate molarity, you can start. molarity describes the relationship between moles of a solute and the volume of a solution. molarity describes the concentration of a solution in moles of solute divided by liters of solution. molarity. Science Geek Molarity.
From www.pinterest.com
Molarity vs Molality How to find the concentration of a solution Science Geek Molarity What is the molarity of. [1] to calculate molarity, you can start. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. molarity describes the relationship between moles of a solute and the. Science Geek Molarity.
From www.pinterest.com
Difference between molarity and normality Chemistry, General Science Geek Molarity molarity describes the concentration of a solution in moles of solute divided by liters of solution. What is the molarity of. Click the start quiz button to proceed. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. the molality refers to the number of moles of a solute per. Science Geek Molarity.
From www.showme.com
Molarity the basics Science, Chemistry ShowMe Science Geek Molarity molarity describes the concentration of a solution in moles of solute divided by liters of solution. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. What is the molarity of. [1] to calculate molarity, you can start. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters. Science Geek Molarity.
From www.youtube.com
How to Calculate Molarity for a Solution YouTube Science Geek Molarity molarity the most common unit of concentration is molarity, which is also the most useful for calculations. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. [1] to calculate molarity, you can start. Masses of solute must first be. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in. Science Geek Molarity.
From mtelec.net
The Amazing Reasons why you should use molarity Science Geek Molarity molarity describes the concentration of a solution in moles of solute divided by liters of solution. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. Masses of solute must first be. What is the molarity of. molar concentration (also called molarity, amount concentration or substance concentration) is a measure. Science Geek Molarity.
From www.showme.com
Molarity Science, Chemistry ShowMe Science Geek Molarity molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. [1] to calculate molarity, you can start. molarity describes the relationship between moles of a. Science Geek Molarity.
From teachsimple.com
Chemistry Molarity Practice by Teach Simple Science Geek Molarity [1] to calculate molarity, you can start. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. What is the molarity of. the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. a. Science Geek Molarity.
From chemistry.stackexchange.com
physical chemistry Temperature dependence of molarity and molality Science Geek Molarity molarity describes the relationship between moles of a solute and the volume of a solution. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. What is the molarity of. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. Click the start. Science Geek Molarity.
From www.youtube.com
Molarity I ( Class XI Chemistry ) by Science Easy Hai. YouTube Science Geek Molarity [1] to calculate molarity, you can start. What is the molarity of. Click the start quiz button to proceed. molarity describes the relationship between moles of a solute and the volume of a solution. Masses of solute must first be. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. . Science Geek Molarity.
From www.showme.com
Calculating molarity of a solution. Science ShowMe Science Geek Molarity molarity describes the concentration of a solution in moles of solute divided by liters of solution. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. What is the molarity of. Masses of solute must first be. [1] to calculate molarity, you can start. a solution of sodium hydroxide, naoh,. Science Geek Molarity.
From www.youtube.com
Molarity of Ions Calculating Concentration of Ions in a Solution Science Geek Molarity molarity describes the concentration of a solution in moles of solute divided by liters of solution. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. [1] to calculate molarity, you can start. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. Click the. Science Geek Molarity.
From sciencestruck.com
Molarity Formula Science Struck Science Geek Molarity What is the molarity of. molarity describes the concentration of a solution in moles of solute divided by liters of solution. molarity describes the relationship between moles of a solute and the volume of a solution. Click the start quiz button to proceed. molarity the most common unit of concentration is molarity, which is also the most. Science Geek Molarity.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Science Geek Molarity What is the molarity of. molarity describes the relationship between moles of a solute and the volume of a solution. Click the start quiz button to proceed. molarity describes the concentration of a solution in moles of solute divided by liters of solution. [1] to calculate molarity, you can start. Masses of solute must first be. molar. Science Geek Molarity.
From www.showme.com
Level I Molarity find the mol Science, Chemistry, molarity ShowMe Science Geek Molarity molarity describes the relationship between moles of a solute and the volume of a solution. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. molarity describes the concentration of a solution in moles of solute divided by liters of solution. What is the molarity of. Click the start quiz button to. Science Geek Molarity.
From ar.inspiredpencil.com
Molarity Science Geek Molarity molarity the most common unit of concentration is molarity, which is also the most useful for calculations. molarity describes the concentration of a solution in moles of solute divided by liters of solution. molarity describes the relationship between moles of a solute and the volume of a solution. Click the start quiz button to proceed. the. Science Geek Molarity.
From www.youtube.com
Difference Between Molarity and Molality Science Hindi Quikr Science Geek Molarity molarity the most common unit of concentration is molarity, which is also the most useful for calculations. [1] to calculate molarity, you can start. What is the molarity of. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. molarity describes the concentration of a solution in moles of solute divided by. Science Geek Molarity.
From www.showme.com
Molarity Science, Chemistry, Solutions ShowMe Science Geek Molarity molarity the most common unit of concentration is molarity, which is also the most useful for calculations. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. [1] to calculate molarity, you can start. What is the molarity of. molarity describes the relationship between moles of a solute and the volume of. Science Geek Molarity.
From www.showme.com
Finding Molarity Science, Chemistry, Stoichiometry ShowMe Science Geek Molarity What is the molarity of. [1] to calculate molarity, you can start. Masses of solute must first be. molarity describes the concentration of a solution in moles of solute divided by liters of solution. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. Click the start quiz button to proceed.. Science Geek Molarity.
From www.showme.com
Level I Molarity find the volume Science, Chemistry, molarity ShowMe Science Geek Molarity a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. [1] to calculate molarity, you can start. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. the molality refers to the number of moles of a solute per mass of a solvent,. Science Geek Molarity.
From www.showme.com
Molarity Science ShowMe Science Geek Molarity Masses of solute must first be. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. Click the start quiz button to proceed. molarity describes the relationship between moles of a solute and the volume of a solution. the molality refers to the number of moles of a solute per mass of. Science Geek Molarity.
From sciencestruck.com
Understanding Molarity and How to Calculate it Easily Science Geek Molarity What is the molarity of. [1] to calculate molarity, you can start. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. Masses of solute must first be. the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles. Science Geek Molarity.
From www.showme.com
Molarity Science ShowMe Science Geek Molarity the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. molarity describes the relationship between moles of a solute and the volume of a solution. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the.. Science Geek Molarity.
From www.youtube.com
Calculating Molarity of Solutions YouTube Science Geek Molarity molarity describes the concentration of a solution in moles of solute divided by liters of solution. molarity describes the relationship between moles of a solute and the volume of a solution. Click the start quiz button to proceed. What is the molarity of. Masses of solute must first be. a solution of sodium hydroxide, naoh, contains 12.0. Science Geek Molarity.
From www.showme.com
Molarity practice worksheet 13 Science, Chemistry, Solutions Science Geek Molarity What is the molarity of. Click the start quiz button to proceed. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. [1] to calculate molarity, you can start. the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the. Science Geek Molarity.
From www.showme.com
Molarity models 13 Science, Chemistry, Solutions Chemistry ShowMe Science Geek Molarity Click the start quiz button to proceed. the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. What is the molarity of. molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. molarity the most. Science Geek Molarity.
From www.youtube.com
Molarity Intro Concentration Calculations for Solutions Straight Science Geek Molarity molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. Click the start quiz button to proceed. What is the molarity of. [1] to calculate molarity, you can start. molarity describes the concentration of a solution in moles of solute divided by liters of solution. Masses of solute must first be. molarity. Science Geek Molarity.
From www.tes.com
Moles, Molarity and Concentration Edexcel 91 Separate (Triple) Science Science Geek Molarity molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the. Masses of solute must first be. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. molarity describes the concentration of a solution in moles of solute divided by liters of solution. a solution. Science Geek Molarity.
From www.pinterest.com
Molarity Easy Science Study chemistry, Chemistry lessons, Easy science Science Geek Molarity a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. molarity describes the concentration of a solution in moles of solute divided by liters of solution. Masses of solute must first be. Click the start quiz button to proceed. molar concentration (also called molarity, amount concentration or substance concentration) is. Science Geek Molarity.
From www.showme.com
Molarity sample problems 1 and 2 Science, Chemistry ShowMe Science Geek Molarity the molality refers to the number of moles of a solute per mass of a solvent, whereas the molarity refers to the number of moles of a. Click the start quiz button to proceed. a solution of sodium hydroxide, naoh, contains 12.0 grams of solute in 2.00 liters of aqueous solution. molar concentration (also called molarity, amount. Science Geek Molarity.
From www.slideserve.com
PPT Molarity PowerPoint Presentation, free download ID3842811 Science Geek Molarity What is the molarity of. molarity describes the relationship between moles of a solute and the volume of a solution. Click the start quiz button to proceed. molarity the most common unit of concentration is molarity, which is also the most useful for calculations. the molality refers to the number of moles of a solute per mass. Science Geek Molarity.