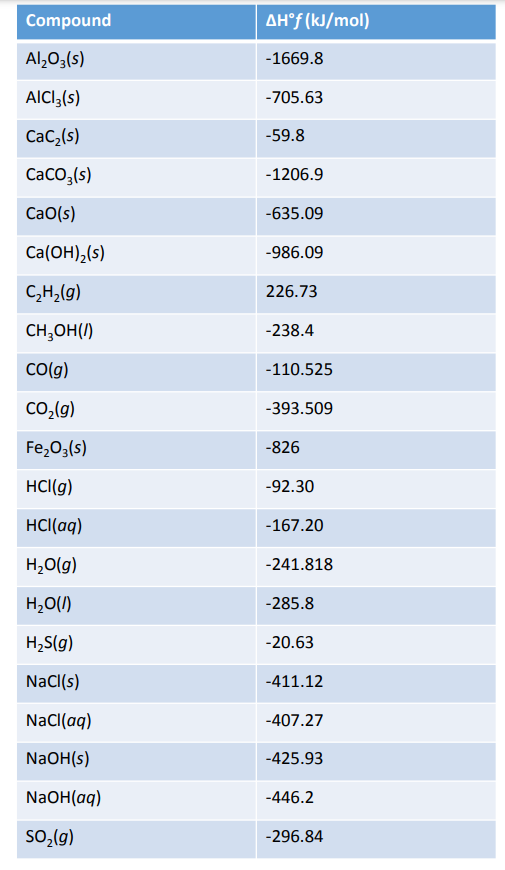
Standard Enthalpy Of Formation N2 . standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of formation of any element in its most stable form is zero by definition. All standard state, 25 °c and 1 bar (written to 1 decimal place). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. The standard enthalpy of formation of any element in its standard state is zero by definition. thermodynamic data at 298 k.
from www.chegg.com
standard enthalpies of formation. The standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. All standard state, 25 °c and 1 bar (written to 1 decimal place). thermodynamic data at 298 k. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. the standard enthalpy of formation of any element in its most stable form is zero by definition.
Solved Using the table of standard enthalpies of formation
Standard Enthalpy Of Formation N2 The standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. thermodynamic data at 298 k. The standard enthalpy of formation of any element in its standard state is zero by definition. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. the standard enthalpy of formation of any element in its most stable form is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. All standard state, 25 °c and 1 bar (written to 1 decimal place). standard enthalpies of formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation N2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. thermodynamic data at 298 k. standard enthalpies of formation. The standard enthalpy of formation of any element in its standard state is zero by definition. All standard state, 25 °c and 1 bar (written to 1 decimal place).. Standard Enthalpy Of Formation N2.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation N2 standard enthalpies of formation. All standard state, 25 °c and 1 bar (written to 1 decimal place). definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. thermodynamic data at 298 k. The standard enthalpy of formation of any element in its standard state is zero by definition. the standard. Standard Enthalpy Of Formation N2.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation N2 All standard state, 25 °c and 1 bar (written to 1 decimal place). definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. standard enthalpies of formation. the standard enthalpy of formation of any element in its most stable form is zero by definition. thermodynamic data at 298 k. For. Standard Enthalpy Of Formation N2.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation N2 For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. thermodynamic data at 298 k. standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. All. Standard Enthalpy Of Formation N2.
From classzonemailmerge.z22.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation N2 The standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. All standard state, 25 °c and 1 bar (written to 1 decimal place). the standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation N2.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation N2 All standard state, 25 °c and 1 bar (written to 1 decimal place). definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. the. Standard Enthalpy Of Formation N2.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation N2 The standard enthalpy of formation of any element in its standard state is zero by definition. standard enthalpies of formation. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. All standard state, 25 °c and 1 bar (written to 1 decimal place). the standard enthalpy of formation is a measure. Standard Enthalpy Of Formation N2.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation N2 standard enthalpies of formation. All standard state, 25 °c and 1 bar (written to 1 decimal place). the standard enthalpy of formation of any element in its most stable form is zero by definition. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. thermodynamic data at 298 k. For. Standard Enthalpy Of Formation N2.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Enthalpy Of Formation N2 The standard enthalpy of formation of any element in its standard state is zero by definition. thermodynamic data at 298 k. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. the standard enthalpy of formation of any element in its most stable form is zero by definition. For example, although. Standard Enthalpy Of Formation N2.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation N2 All standard state, 25 °c and 1 bar (written to 1 decimal place). standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. For example, although oxygen can. Standard Enthalpy Of Formation N2.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation N2 All standard state, 25 °c and 1 bar (written to 1 decimal place). the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. thermodynamic data at 298 k. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is. Standard Enthalpy Of Formation N2.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation N2 The standard enthalpy of formation of any element in its standard state is zero by definition. thermodynamic data at 298 k. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2),. Standard Enthalpy Of Formation N2.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation N2 standard enthalpies of formation. the standard enthalpy of formation of any element in its most stable form is zero by definition. thermodynamic data at 298 k. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation of any element in its standard state is zero by definition. For example,. Standard Enthalpy Of Formation N2.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation N2 thermodynamic data at 298 k. the standard enthalpy of formation of any element in its most stable form is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. The standard enthalpy of formation of any element in its standard state is zero by definition.. Standard Enthalpy Of Formation N2.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation N2 definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1. Standard Enthalpy Of Formation N2.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation N2 definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. thermodynamic data at 298 k. the standard enthalpy of formation of any element in its most stable form is zero by. Standard Enthalpy Of Formation N2.
From www.toppr.com
The enthalpy of formation of ammonia is −46.0 kJ mol−1. The enthalpy Standard Enthalpy Of Formation N2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. the standard enthalpy of formation of any element in its most stable form is zero by definition. standard enthalpies of formation.. Standard Enthalpy Of Formation N2.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation N2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. All standard state, 25 °c and 1 bar (written to 1 decimal place). standard enthalpies of formation. the standard enthalpy of formation of any element in its most stable form is zero by definition. thermodynamic data at. Standard Enthalpy Of Formation N2.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation N2 thermodynamic data at 298 k. the standard enthalpy of formation of any element in its most stable form is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. The standard enthalpy of formation of any element. Standard Enthalpy Of Formation N2.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation N2 definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. All standard state, 25 °c and 1 bar (written to 1 decimal place). standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of. Standard Enthalpy Of Formation N2.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation N2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. thermodynamic data at 298 k. the standard enthalpy of formation of any element in its most stable form is zero by definition. All standard state, 25 °c and 1 bar (written to 1 decimal place). standard enthalpies. Standard Enthalpy Of Formation N2.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation N2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most. Standard Enthalpy Of Formation N2.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation N2 For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. the standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation N2.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation N2 All standard state, 25 °c and 1 bar (written to 1 decimal place). standard enthalpies of formation. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at. Standard Enthalpy Of Formation N2.
From www.chegg.com
Solved Table 1. Standard enthalpies of formation for Standard Enthalpy Of Formation N2 the standard enthalpy of formation of any element in its most stable form is zero by definition. All standard state, 25 °c and 1 bar (written to 1 decimal place). definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. thermodynamic data at 298 k. The standard enthalpy of formation of. Standard Enthalpy Of Formation N2.
From www.youtube.com
5.7 Standard Enthalpies of Formation YouTube Standard Enthalpy Of Formation N2 thermodynamic data at 298 k. the standard enthalpy of formation of any element in its most stable form is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation N2.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation N2 thermodynamic data at 298 k. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. The standard enthalpy of formation of any element in its standard state is zero by definition. definition and explanation of the terms standard state and. Standard Enthalpy Of Formation N2.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation N2 All standard state, 25 °c and 1 bar (written to 1 decimal place). definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. The standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation N2.
From exoxhphey.blob.core.windows.net
Standard Enthalpy Of Formation Calculator at Susan blog Standard Enthalpy Of Formation N2 definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and. Standard Enthalpy Of Formation N2.
From schoolworkhelper.net
StandardEnthalpiesFormation Standard Enthalpy Of Formation N2 the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. standard enthalpies of formation. The standard enthalpy of formation of any element in its standard state is zero by definition. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. thermodynamic. Standard Enthalpy Of Formation N2.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation N2 All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. the standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation N2.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation N2 For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. All standard state, 25 °c and 1 bar (written to 1 decimal place). standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or. Standard Enthalpy Of Formation N2.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation N2 All standard state, 25 °c and 1 bar (written to 1 decimal place). definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. The standard. Standard Enthalpy Of Formation N2.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Of Formation N2 definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. the standard enthalpy of formation of any element in its most stable form is zero by definition. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o. Standard Enthalpy Of Formation N2.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation N2 For example, although oxygen can exist as ozone (o 3), atomic oxygen (o), and molecular oxygen (o 2), o 2 is the most stable form at 1 atm. standard enthalpies of formation. thermodynamic data at 298 k. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation of any element. Standard Enthalpy Of Formation N2.