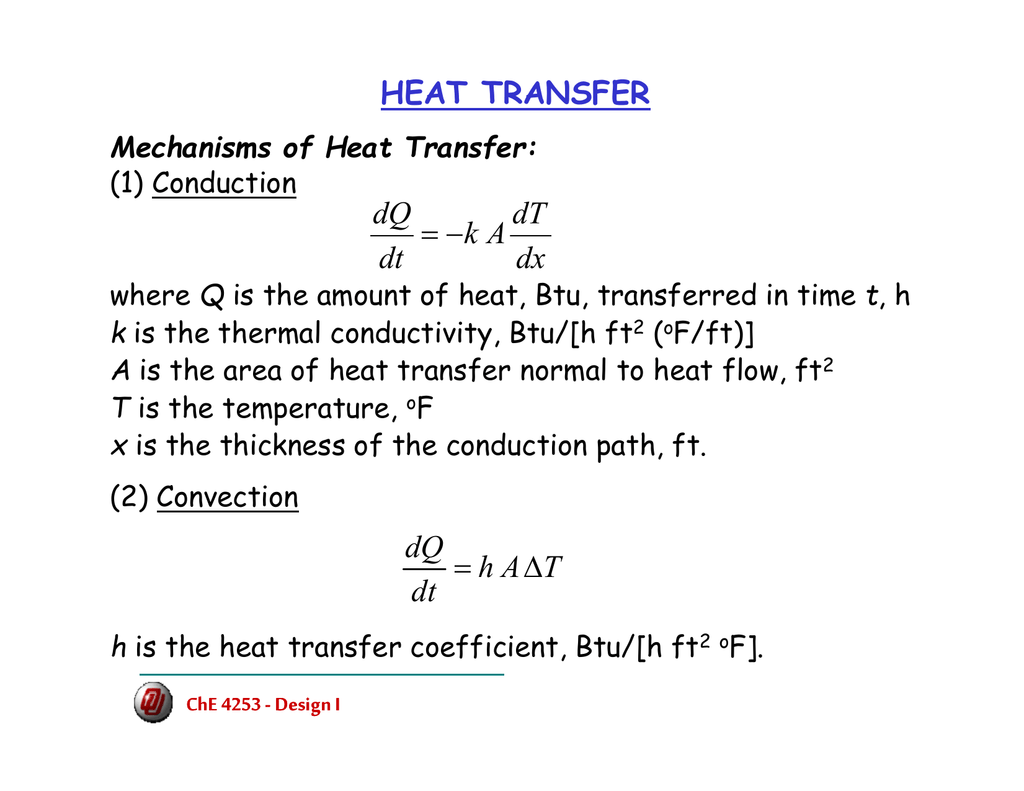
What Is Heat Transfer Equation . the following equation relates to the heat transferred from one system to another. \ [q = mc\delta t,\] where \ (q\) is the symbol for. the equation for heat transfer q is. with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. Q = m c δ t, q = m c δ t, 11.7. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the quantitative relationship between heat transfer and temperature change contains all three factors: use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. Where m is the mass of the substance and δ t is the change in its. \ (\begin {array} {l}q= c\times m\times.
from mungfali.com
the quantitative relationship between heat transfer and temperature change contains all three factors: the following equation relates to the heat transferred from one system to another. Where m is the mass of the substance and δ t is the change in its. with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. the equation for heat transfer q is. Q = m c δ t, q = m c δ t, 11.7. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. \ (\begin {array} {l}q= c\times m\times. \ [q = mc\delta t,\] where \ (q\) is the symbol for.
Heat Transfer Coefficient Equation
What Is Heat Transfer Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. \ (\begin {array} {l}q= c\times m\times. Where m is the mass of the substance and δ t is the change in its. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the quantitative relationship between heat transfer and temperature change contains all three factors: \ [q = mc\delta t,\] where \ (q\) is the symbol for. Q = m c δ t, q = m c δ t, 11.7. the following equation relates to the heat transferred from one system to another. the equation for heat transfer q is. with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from.
From www.slideshare.net
Thermodynamics Chapter 3 Heat Transfer What Is Heat Transfer Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in. What Is Heat Transfer Equation.
From www.youtube.com
heat conduction equation in Cartesian coordinates in general BASICS OF What Is Heat Transfer Equation the quantitative relationship between heat transfer and temperature change contains all three factors: with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. the equation for heat transfer q is. \ (\begin {array} {l}q= c\times m\times. use the equation for heat transfer q. What Is Heat Transfer Equation.
From www.slideserve.com
PPT Heat Transfer Coefficient PowerPoint Presentation, free download What Is Heat Transfer Equation \ [q = mc\delta t,\] where \ (q\) is the symbol for. the quantitative relationship between heat transfer and temperature change contains all three factors: use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. Q =. What Is Heat Transfer Equation.
From www.grc.nasa.gov
Heat Transfer What Is Heat Transfer Equation with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. \ [q. What Is Heat Transfer Equation.
From www.slideserve.com
PPT Overall Heat Transfer Coefficient PowerPoint Presentation, free What Is Heat Transfer Equation the equation for heat transfer q is. Q = m c δ t, q = m c δ t, 11.7. the following equation relates to the heat transferred from one system to another. with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. \. What Is Heat Transfer Equation.
From www.youtube.com
Heat Transfer Chapter 2 Example Problem 6 Solving the Heat What Is Heat Transfer Equation Q = m c δ t, q = m c δ t, 11.7. Where m is the mass of the substance and δ t is the change in its. the quantitative relationship between heat transfer and temperature change contains all three factors: use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by. What Is Heat Transfer Equation.
From www.slideserve.com
PPT 1D, Steady State Heat Transfer with Heat Generation Fins and What Is Heat Transfer Equation the following equation relates to the heat transferred from one system to another. with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. Q = m c δ t, q = m c δ t, 11.7. \ [q = mc\delta t,\] where \ (q\) is. What Is Heat Transfer Equation.
From www.youtube.com
Heat Transfer L12 p1 Finite Difference Heat Equation YouTube What Is Heat Transfer Equation \ [q = mc\delta t,\] where \ (q\) is the symbol for. the equation for heat transfer q is. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. Q = m c δ t,. What Is Heat Transfer Equation.
From www.slideserve.com
PPT SECTION 1 HEAT TRANSFER ANALYSIS PowerPoint Presentation, free What Is Heat Transfer Equation with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. Where m is the mass of the substance and δ t is the change in its. Q = m c δ t, q = m c δ t, 11.7. the following equation relates to the. What Is Heat Transfer Equation.
From www.youtube.com
Heat Transfer L22 p3 Bulk Temperature Constant Heat Flux YouTube What Is Heat Transfer Equation Where m is the mass of the substance and δ t is the change in its. the quantitative relationship between heat transfer and temperature change contains all three factors: the following equation relates to the heat transferred from one system to another. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost. What Is Heat Transfer Equation.
From studylib.net
Heat Equation What Is Heat Transfer Equation the quantitative relationship between heat transfer and temperature change contains all three factors: \ (\begin {array} {l}q= c\times m\times. Where m is the mass of the substance and δ t is the change in its. the equation for heat transfer q is. the following equation relates to the heat transferred from one system to another. with. What Is Heat Transfer Equation.
From www.youtube.com
Heat Transfer L2 p2 Convection Rate Equation Newton's Law of What Is Heat Transfer Equation use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. Where m. What Is Heat Transfer Equation.
From studylib.net
What is Heat Transfer? What Is Heat Transfer Equation Where m is the mass of the substance and δ t is the change in its. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. use the equation for heat transfer q = m c δ. What Is Heat Transfer Equation.
From www.slideserve.com
PPT Heat and the First Law of Thermodynamics PowerPoint Presentation What Is Heat Transfer Equation the following equation relates to the heat transferred from one system to another. the quantitative relationship between heat transfer and temperature change contains all three factors: Q = m c δ t, q = m c δ t, 11.7. Where m is the mass of the substance and δ t is the change in its. use the. What Is Heat Transfer Equation.
From www.youtube.com
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi What Is Heat Transfer Equation use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. \ [q = mc\delta t,\] where \ (q\) is the symbol for. Q = m c δ t, q = m c δ t, 11.7. Where. What Is Heat Transfer Equation.
From www.slideserve.com
PPT Heat Transfer Physical Origins and Rate Equations PowerPoint What Is Heat Transfer Equation the following equation relates to the heat transferred from one system to another. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. \ (\begin {array} {l}q= c\times m\times. Where m is the mass of the substance. What Is Heat Transfer Equation.
From www.slideserve.com
PPT SECTION 1 HEAT TRANSFER ANALYSIS PowerPoint Presentation, free What Is Heat Transfer Equation Q = m c δ t, q = m c δ t, 11.7. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. \ [q = mc\delta t,\] where \ (q\) is the symbol for. the following. What Is Heat Transfer Equation.
From mungfali.com
Heat Transfer Coefficient Equation What Is Heat Transfer Equation the following equation relates to the heat transferred from one system to another. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. Q = m c δ t, q = m c δ t, 11.7. . What Is Heat Transfer Equation.
From www.slideserve.com
PPT Heat Transfer PowerPoint Presentation, free download ID2247219 What Is Heat Transfer Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. Where m is the mass of the substance and δ t is the change in its. with a temperature difference of 15°, the amount of heat conducted. What Is Heat Transfer Equation.
From www.youtube.com
General heat conduction equation YouTube What Is Heat Transfer Equation \ [q = mc\delta t,\] where \ (q\) is the symbol for. the quantitative relationship between heat transfer and temperature change contains all three factors: \ (\begin {array} {l}q= c\times m\times. Where m is the mass of the substance and δ t is the change in its. Q = m c δ t, q = m c δ t,. What Is Heat Transfer Equation.
From www.youtube.com
Heat Transfer L14 p2 Heat Equation Transient Solution YouTube What Is Heat Transfer Equation Q = m c δ t, q = m c δ t, 11.7. the following equation relates to the heat transferred from one system to another. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. . What Is Heat Transfer Equation.
From www.tessshebaylo.com
Equation For Heat Transfer By Radiation Tessshebaylo What Is Heat Transfer Equation the quantitative relationship between heat transfer and temperature change contains all three factors: \ (\begin {array} {l}q= c\times m\times. Where m is the mass of the substance and δ t is the change in its. \ [q = mc\delta t,\] where \ (q\) is the symbol for. use the equation for heat transfer q = m c δ. What Is Heat Transfer Equation.
From www.chemicalslearning.com
Fourier's Law of Heat Conduction, Formula and Derivation What Is Heat Transfer Equation use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. Where m is the mass of the substance and δ t is the change in its. use the equation for heat transfer \(q = mc\delta. What Is Heat Transfer Equation.
From www.slideserve.com
PPT SECTION 1 HEAT TRANSFER ANALYSIS PowerPoint Presentation, free What Is Heat Transfer Equation the equation for heat transfer q is. \ [q = mc\delta t,\] where \ (q\) is the symbol for. the quantitative relationship between heat transfer and temperature change contains all three factors: use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the. What Is Heat Transfer Equation.
From www.youtube.com
Heat Transfer U2L4 General Heat Conduction Equation 1 YouTube What Is Heat Transfer Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the equation for heat transfer q is. \ [q = mc\delta t,\] where \ (q\) is the symbol for. Q = m c δ t, q =. What Is Heat Transfer Equation.
From www.youtube.com
What Does It Mean to Solve the Heat Equation PDE? An Introduction with What Is Heat Transfer Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the equation for heat transfer q is. \ [q = mc\delta t,\] where \ (q\) is the symbol for. \ (\begin {array} {l}q= c\times m\times. Where m. What Is Heat Transfer Equation.
From www.slideserve.com
PPT Heat Transfer Physical Origins and Rate Equations PowerPoint What Is Heat Transfer Equation with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. \ (\begin {array} {l}q= c\times m\times. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific. What Is Heat Transfer Equation.
From www.slideserve.com
PPT Chapter 1 Fourier Equation and Thermal Conductivity PowerPoint What Is Heat Transfer Equation use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. Q = m c δ t, q = m c δ t, 11.7. with a temperature difference of 15°, the amount of heat conducted through the aluminum. What Is Heat Transfer Equation.
From www.engproguides.com
Heat Transfer Key Equations for the Mechanical PE Exam What Is Heat Transfer Equation with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. the equation for heat transfer q is. the following equation relates to the heat transferred from one system to another. \ [q = mc\delta t,\] where \ (q\) is the symbol for. Q =. What Is Heat Transfer Equation.
From www.youtube.com
Example of Heat Transfer calculation part 1 YouTube What Is Heat Transfer Equation with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. the following equation relates to the heat transferred from one system to another. the equation for heat transfer q is. use the equation for heat transfer \(q = mc\delta t\) to express the. What Is Heat Transfer Equation.
From study.com
Radiation Heat Transfer Examples, Equation & Formula Video & Lesson What Is Heat Transfer Equation \ [q = mc\delta t,\] where \ (q\) is the symbol for. with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. \ (\begin {array} {l}q= c\times m\times. Where m is the mass of the substance and δ t is the change in its. the. What Is Heat Transfer Equation.
From www.slideshare.net
11 Heat Transfer What Is Heat Transfer Equation the quantitative relationship between heat transfer and temperature change contains all three factors: the following equation relates to the heat transferred from one system to another. the equation for heat transfer q is. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred. What Is Heat Transfer Equation.
From www.youtube.com
Heat Transfer L1 p4 Conduction Rate Equation Fourier's Law YouTube What Is Heat Transfer Equation \ (\begin {array} {l}q= c\times m\times. with a temperature difference of 15°, the amount of heat conducted through the aluminum per second per square meter can be calculated from. \ [q = mc\delta t,\] where \ (q\) is the symbol for. Where m is the mass of the substance and δ t is the change in its. use. What Is Heat Transfer Equation.
From heattransferkarikuse.blogspot.com
Heat Transfer Heat Transfer Q Equation What Is Heat Transfer Equation Q = m c δ t, q = m c δ t, 11.7. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. the following equation relates to the heat transferred from one system to another. . What Is Heat Transfer Equation.
From slidetodoc.com
CONVECTION Convection Heat Transfer Why is it windy What Is Heat Transfer Equation the quantitative relationship between heat transfer and temperature change contains all three factors: \ (\begin {array} {l}q= c\times m\times. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. the following equation relates to. What Is Heat Transfer Equation.