Buffer Lab Answers . The professor used naoh (a base) to raise the ph to the desired value. To accomplish this, cells contain buffers. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. Explain the correct method for. A buffer has a limited capacity to prevent ph from changing drastically in response. Ka = 1.8 x 10. Which reagent was used to adjust the ph of the buffer solution? Buffers are most effective at protecting against ph change when they have similar concentrations of both components of the weak acid/conjugate. Compare the results of the ph sensitivity data in parts a and c. Designing and preparing a buffer. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Explain why the buffer capacity depends on the concentration of the buffer. Calculate the ph of an unbuffered 0.010m acetic acid solution. This is important for living. In particular, you will learn to:
from www.chegg.com
Explain the correct method for. Explain why the buffer capacity depends on the concentration of the buffer. Buffers are most effective at protecting against ph change when they have similar concentrations of both components of the weak acid/conjugate. A buffer has a limited capacity to prevent ph from changing drastically in response. Which reagent was used to adjust the ph of the buffer solution? The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. This is important for living. A ph buffer resists the change in ph when acids or bases are added to a solution or as conditions shift. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. In particular, you will learn to:
Solved Acids Bases, pH, and Buffers Lab Information 21/ hr
Buffer Lab Answers Explain the correct method for. This is important for living. A ph buffer resists the change in ph when acids or bases are added to a solution or as conditions shift. Which reagent was used to adjust the ph of the buffer solution? In particular, you will learn to: Designing and preparing a buffer. The professor used naoh (a base) to raise the ph to the desired value. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Ka = 1.8 x 10. A buffer has a limited capacity to prevent ph from changing drastically in response. Buffers are most effective at protecting against ph change when they have similar concentrations of both components of the weak acid/conjugate. Calculate the ph of an unbuffered 0.010m acetic acid solution. Compare the results of the ph sensitivity data in parts a and c. Explain why the buffer capacity depends on the concentration of the buffer. To accomplish this, cells contain buffers. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain.
From www.chegg.com
Solved BUFFERS · BUFFERS AND BUFFER CAPACITY . INTRODUCTION Buffer Lab Answers 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. Explain why the buffer capacity depends on the concentration of the buffer. To accomplish this, cells contain buffers. In particular, you will learn to: Buffers are most effective at protecting against ph change when they have similar concentrations of. Buffer Lab Answers.
From www.chegg.com
Solved Acids, Bases, Buffers and p Postlaboratory Buffer Lab Answers A buffer has a limited capacity to prevent ph from changing drastically in response. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. Buffers are most effective at protecting against ph change when they have similar concentrations of both components of the weak acid/conjugate. Calculate the ph of. Buffer Lab Answers.
From www.chegg.com
Solved Buffer lab Buffer Lab Answers This is important for living. To accomplish this, cells contain buffers. In particular, you will learn to: The professor used naoh (a base) to raise the ph to the desired value. Ka = 1.8 x 10. Which reagent was used to adjust the ph of the buffer solution? A ph buffer resists the change in ph when acids or bases. Buffer Lab Answers.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog Buffer Lab Answers Calculate the ph of an unbuffered 0.010m acetic acid solution. Compare the results of the ph sensitivity data in parts a and c. The professor used naoh (a base) to raise the ph to the desired value. In particular, you will learn to: Buffers are most effective at protecting against ph change when they have similar concentrations of both components. Buffer Lab Answers.
From www.coursehero.com
[Solved] Buffer Lab Answers A ph buffer resists the change in ph when acids or bases are added to a solution or as conditions shift. Explain why the buffer capacity depends on the concentration of the buffer. This is important for living. To accomplish this, cells contain buffers. The professor used naoh (a base) to raise the ph to the desired value. Calculate the. Buffer Lab Answers.
From www.chegg.com
Solved Buffer lab Buffer Lab Answers A buffer has a limited capacity to prevent ph from changing drastically in response. Which reagent was used to adjust the ph of the buffer solution? Ka = 1.8 x 10. Designing and preparing a buffer. This is important for living. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where. Buffer Lab Answers.
From www.studocu.com
CHM 111 Experiment 5 Lab filled in CHM 111 Lab 5 Chemical Buffer Lab Answers The professor used naoh (a base) to raise the ph to the desired value. A ph buffer resists the change in ph when acids or bases are added to a solution or as conditions shift. Which reagent was used to adjust the ph of the buffer solution? Calculate the ph of an unbuffered 0.010m acetic acid solution. To accomplish this,. Buffer Lab Answers.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffer Lab Answers In particular, you will learn to: Explain the correct method for. Calculate the ph of an unbuffered 0.010m acetic acid solution. The professor used naoh (a base) to raise the ph to the desired value. To accomplish this, cells contain buffers. This is important for living. Which reagent was used to adjust the ph of the buffer solution? 1) distinguish. Buffer Lab Answers.
From studylib.net
Buffer Capacity Buffer Lab Answers Explain why the buffer capacity depends on the concentration of the buffer. In particular, you will learn to: This is important for living. Designing and preparing a buffer. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. The dissociation of a weak acid in water yields h+ ion. Buffer Lab Answers.
From www.studypool.com
SOLUTION Chm 115l buffers lab worksheet gcu Studypool Buffer Lab Answers To accomplish this, cells contain buffers. Compare the results of the ph sensitivity data in parts a and c. Explain why the buffer capacity depends on the concentration of the buffer. Calculate the ph of an unbuffered 0.010m acetic acid solution. The professor used naoh (a base) to raise the ph to the desired value. This is important for living.. Buffer Lab Answers.
From www.chegg.com
Solved pH and Buffer lab reportI need help with question Buffer Lab Answers The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. The professor used naoh (a base) to raise the ph to the desired value. Which reagent was used to. Buffer Lab Answers.
From www.chegg.com
PreLab 11 BUFFER AND BUFFER CAPACITY Record ALL Buffer Lab Answers The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. A ph buffer resists the change in ph when acids or bases are added to a solution or as conditions shift. Designing and preparing a buffer. Explain why the buffer capacity depends on the concentration of the buffer. To accomplish. Buffer Lab Answers.
From www.chegg.com
Solved for a buffer lab, a buffer with ph 7.4 was prepared Buffer Lab Answers The professor used naoh (a base) to raise the ph to the desired value. Compare the results of the ph sensitivity data in parts a and c. This is important for living. Explain the correct method for. In particular, you will learn to: Calculate the ph of an unbuffered 0.010m acetic acid solution. Explain why the buffer capacity depends on. Buffer Lab Answers.
From www.chegg.com
student lab Buffers.pdf Buffers Keep the Balance Buffer Lab Answers A ph buffer resists the change in ph when acids or bases are added to a solution or as conditions shift. Which reagent was used to adjust the ph of the buffer solution? Calculate the ph of an unbuffered 0.010m acetic acid solution. Ka = 1.8 x 10. This is important for living. Designing and preparing a buffer. Buffers are. Buffer Lab Answers.
From www.studypool.com
SOLUTION Chm 115l buffers lab worksheet gcu Studypool Buffer Lab Answers Calculate the ph of an unbuffered 0.010m acetic acid solution. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Designing and preparing a buffer. Which reagent was used to adjust the ph of the buffer solution? This is important for living. Explain why the buffer capacity depends on the. Buffer Lab Answers.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Lab Answers Ka = 1.8 x 10. Which reagent was used to adjust the ph of the buffer solution? To accomplish this, cells contain buffers. Designing and preparing a buffer. Explain why the buffer capacity depends on the concentration of the buffer. The professor used naoh (a base) to raise the ph to the desired value. Buffers are most effective at protecting. Buffer Lab Answers.
From www.chegg.com
Solved Exp 25. pH MeasurementsBuffers and Their Properties. Buffer Lab Answers To accomplish this, cells contain buffers. Explain the correct method for. In particular, you will learn to: The professor used naoh (a base) to raise the ph to the desired value. Compare the results of the ph sensitivity data in parts a and c. A buffer has a limited capacity to prevent ph from changing drastically in response. Explain why. Buffer Lab Answers.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A Buffer Lab Answers A ph buffer resists the change in ph when acids or bases are added to a solution or as conditions shift. Designing and preparing a buffer. Which reagent was used to adjust the ph of the buffer solution? A buffer has a limited capacity to prevent ph from changing drastically in response. The dissociation of a weak acid in water. Buffer Lab Answers.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points Buffer Lab Answers Which reagent was used to adjust the ph of the buffer solution? A buffer has a limited capacity to prevent ph from changing drastically in response. Ka = 1.8 x 10. This is important for living. In particular, you will learn to: Explain the correct method for. The dissociation of a weak acid in water yields h+ ion and the. Buffer Lab Answers.
From www.chegg.com
Solved Chemistry 1051 Laboratory BUFFERS acidic PART 2 Buffer Lab Answers Designing and preparing a buffer. Explain the correct method for. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. To accomplish this, cells contain buffers. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. Compare the results. Buffer Lab Answers.
From www.chegg.com
Solved PreLab Investigation 11 How does the buffer Buffer Lab Answers A buffer has a limited capacity to prevent ph from changing drastically in response. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Ka = 1.8 x 10.. Buffer Lab Answers.
From www.numerade.com
SOLVED Preparation of Buffer Solutions EXPERIMENT 2 PREPARING A Buffer Lab Answers Compare the results of the ph sensitivity data in parts a and c. To accomplish this, cells contain buffers. Explain why the buffer capacity depends on the concentration of the buffer. The professor used naoh (a base) to raise the ph to the desired value. Calculate the ph of an unbuffered 0.010m acetic acid solution. This is important for living.. Buffer Lab Answers.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog Buffer Lab Answers The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. In particular, you will learn to: Buffers are most effective at protecting against ph change when they have similar concentrations of both components of the weak acid/conjugate. Explain why the buffer capacity depends on the concentration of the buffer. The. Buffer Lab Answers.
From www.chegg.com
Solved Experiment VII Buffers Lab Report ( 49 pts) I. Buffer Lab Answers Calculate the ph of an unbuffered 0.010m acetic acid solution. Which reagent was used to adjust the ph of the buffer solution? A buffer has a limited capacity to prevent ph from changing drastically in response. The professor used naoh (a base) to raise the ph to the desired value. Buffers are most effective at protecting against ph change when. Buffer Lab Answers.
From www.chegg.com
University of Massachusetts Boston hemistry 118 Buffer Lab Answers Which reagent was used to adjust the ph of the buffer solution? 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Explain the correct method for. Compare the. Buffer Lab Answers.
From www.numerade.com
SOLVED Buffers Observing the Effect of Adding Acid or Base In this Buffer Lab Answers The professor used naoh (a base) to raise the ph to the desired value. A ph buffer resists the change in ph when acids or bases are added to a solution or as conditions shift. Explain why the buffer capacity depends on the concentration of the buffer. Which reagent was used to adjust the ph of the buffer solution? The. Buffer Lab Answers.
From studylib.net
Expt 4 Buffer Lab Answers Buffer Lab Answers Calculate the ph of an unbuffered 0.010m acetic acid solution. Explain why the buffer capacity depends on the concentration of the buffer. To accomplish this, cells contain buffers. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. Buffers are most effective at protecting against ph change when they. Buffer Lab Answers.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Lab Answers Explain the correct method for. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Designing and preparing a buffer. To accomplish this, cells contain buffers. Which reagent was used to adjust the ph of the buffer solution? Buffers are most effective at protecting against ph change when they have. Buffer Lab Answers.
From www.studocu.com
Acids, Bases, and p H Buffers SA answers Drylab Acids, Bases, and Buffer Lab Answers Explain why the buffer capacity depends on the concentration of the buffer. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. A buffer has a limited capacity to prevent ph from changing drastically in response. This is important for living. Buffers are most effective at protecting against ph. Buffer Lab Answers.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at Buffer Lab Answers To accomplish this, cells contain buffers. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. This is important for living. Which reagent was used to adjust the ph of the buffer solution? Explain the correct method for. A buffer has a limited capacity to prevent ph from changing. Buffer Lab Answers.
From ceirptus.blob.core.windows.net
What Is Buffer Solution In Chemistry at Elizabeth Olsen blog Buffer Lab Answers Compare the results of the ph sensitivity data in parts a and c. Explain why the buffer capacity depends on the concentration of the buffer. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. A buffer has a limited capacity to prevent ph from changing drastically in response.. Buffer Lab Answers.
From www.chegg.com
Solved Acids Bases, pH, and Buffers Lab Information 21/ hr Buffer Lab Answers Calculate the ph of an unbuffered 0.010m acetic acid solution. Explain the correct method for. A ph buffer resists the change in ph when acids or bases are added to a solution or as conditions shift. In particular, you will learn to: 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer. Buffer Lab Answers.
From www.chegg.com
Solved 41 Experiment 5 Buffer Lab The completed data Buffer Lab Answers To accomplish this, cells contain buffers. Buffers are most effective at protecting against ph change when they have similar concentrations of both components of the weak acid/conjugate. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. Explain the correct method for. Which reagent was used to adjust the ph. Buffer Lab Answers.
From www.studocu.com
Chemistry of Life p H and Buffers Chemistry of Life pH and Buffers Buffer Lab Answers Explain the correct method for. In particular, you will learn to: The professor used naoh (a base) to raise the ph to the desired value. 1) distinguish between strong acid and weak acid systems, 2) define a buffer and explain how a buffer works, 3) explain. Designing and preparing a buffer. Ka = 1.8 x 10. Which reagent was used. Buffer Lab Answers.
From www.chegg.com
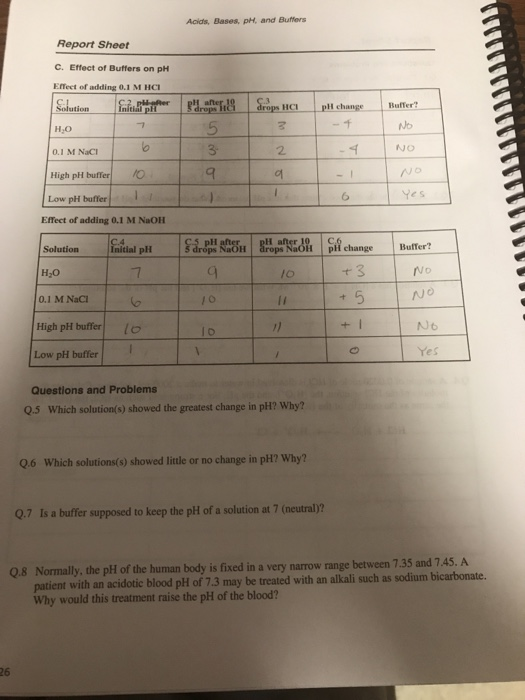
Solved REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 PH Buffer Lab Answers Explain the correct method for. Designing and preparing a buffer. In particular, you will learn to: Ka = 1.8 x 10. The dissociation of a weak acid in water yields h+ ion and the conjugate base of the acid, where the. To accomplish this, cells contain buffers. A ph buffer resists the change in ph when acids or bases are. Buffer Lab Answers.