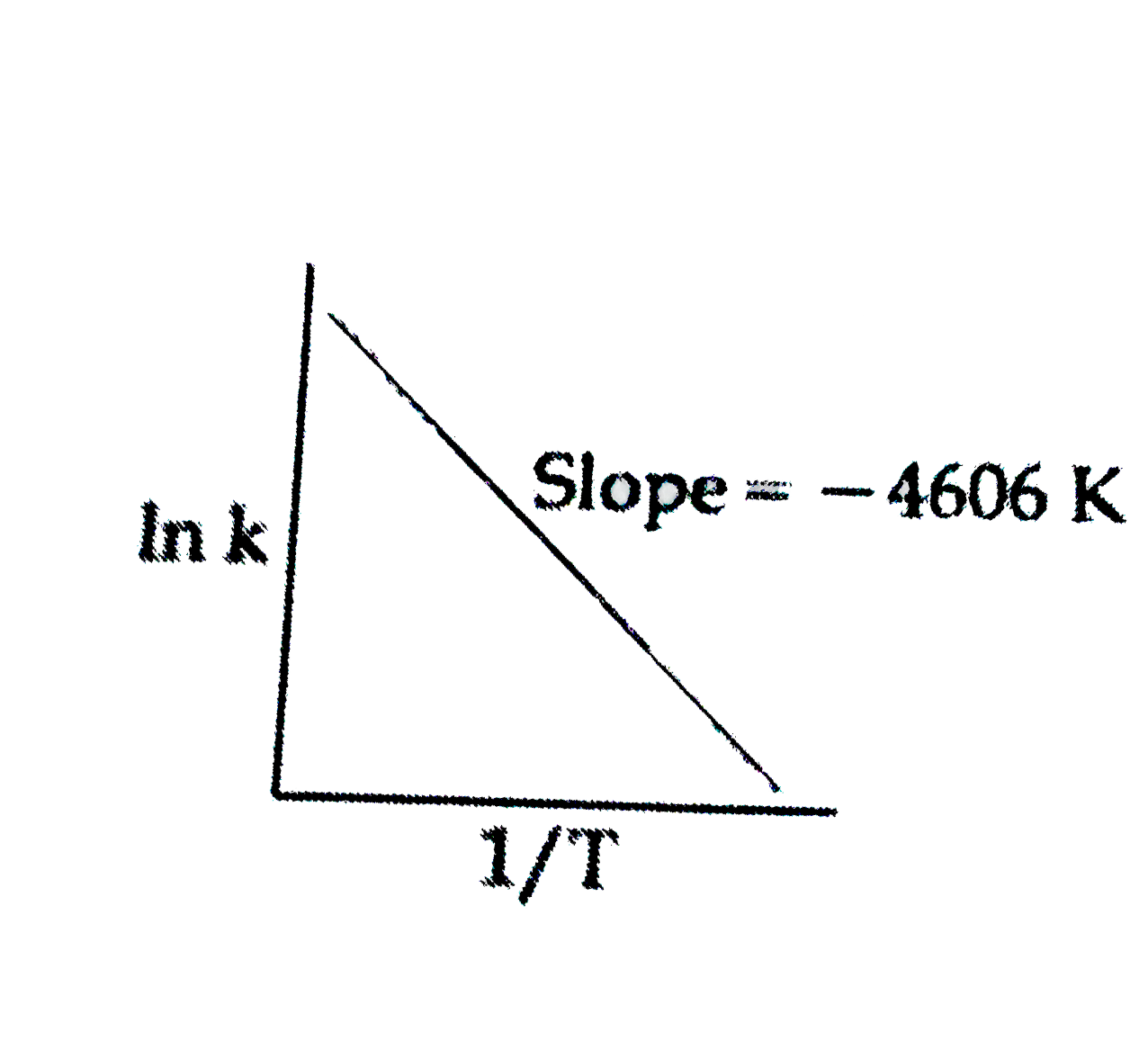The Rate Constant K Is Given By . The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: K(t) is the reaction rate constant that depends on temperature. Therefore, the units of k (assuming that. Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the. [b] is the molar concentrations of. [a] is the molar concentrations of substances a in moles per unit volume of solution. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. K = rate/[a] x [b] y. Rearranging the rate equation, the value of the rate constant ‘k’ is given by: A shortcut to determining the units of rate constant. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this.
from www.doubtnut.com
[a] is the molar concentrations of substances a in moles per unit volume of solution. Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the. Rearranging the rate equation, the value of the rate constant ‘k’ is given by: K = rate/[a] x [b] y. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. K(t) is the reaction rate constant that depends on temperature. A shortcut to determining the units of rate constant. The rate constant for the first order decomposition of h 2 o 2 is given by the following equation:
For a reaction, consider the plot of In K versus 1//T given in the fig
The Rate Constant K Is Given By The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. K = rate/[a] x [b] y. Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the. [a] is the molar concentrations of substances a in moles per unit volume of solution. Rearranging the rate equation, the value of the rate constant ‘k’ is given by: Therefore, the units of k (assuming that. [b] is the molar concentrations of. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. K(t) is the reaction rate constant that depends on temperature. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. A shortcut to determining the units of rate constant. The rate constant for the first order decomposition of h 2 o 2 is given by the following equation:
From www.numerade.com
SOLVED The rate constant of a reaction is measured at different The Rate Constant K Is Given By The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: [a] is the molar concentrations of substances a in moles per unit volume of solution. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. [b]. The Rate Constant K Is Given By.
From www.youtube.com
16.2 Effect of temperature on the rate constant k (HL) YouTube The Rate Constant K Is Given By A shortcut to determining the units of rate constant. [b] is the molar concentrations of. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. Rearranging the rate equation, the value of the rate constant ‘k’ is given by: [a] is the molar concentrations of substances a in. The Rate Constant K Is Given By.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free The Rate Constant K Is Given By The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: Therefore, the units of k (assuming that. [a] is the molar concentrations of substances a in. The Rate Constant K Is Given By.
From study.com
Identifying HalfLife Given the Rate Constant Chemistry The Rate Constant K Is Given By The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. A shortcut to determining the units of rate constant. [b] is the molar concentrations of. K(t) is the reaction rate constant that depends on temperature. Notice how for each order, we determined the units of. The Rate Constant K Is Given By.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature The Rate Constant K Is Given By K(t) is the reaction rate constant that depends on temperature. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. A shortcut to determining the units of rate constant. The. The Rate Constant K Is Given By.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube The Rate Constant K Is Given By The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. Therefore, the units of k (assuming that. The rate constant k and the reaction orders m and n must be determined experimentally by. The Rate Constant K Is Given By.
From www.youtube.com
units of the rate constant k derivations YouTube The Rate Constant K Is Given By The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Therefore, the units of k (assuming that. K = rate/[a] x [b] y. [b] is the. The Rate Constant K Is Given By.
From www.storyofmathematics.com
Rate Constant Calculator + Online Solver With Free Steps The Rate Constant K Is Given By A shortcut to determining the units of rate constant. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. Therefore, the units of k (assuming that. The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: K(t) is the. The Rate Constant K Is Given By.
From www.numerade.com
SOLVED Use the experimental data given in the table and determine the The Rate Constant K Is Given By The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. [b] is the molar concentrations of. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. K(t) is the reaction rate constant that depends on temperature. A. The Rate Constant K Is Given By.
From www.youtube.com
How to Determine Units of Rate Constant k (Shortcut with Examples The Rate Constant K Is Given By K(t) is the reaction rate constant that depends on temperature. Rearranging the rate equation, the value of the rate constant ‘k’ is given by: K = rate/[a] x [b] y. [a] is the molar concentrations of substances a in moles per unit volume of solution. Therefore, the units of k (assuming that. A shortcut to determining the units of rate. The Rate Constant K Is Given By.
From byjus.com
According to Arrhenius equation rate constant k is equal to A · e E a The Rate Constant K Is Given By A shortcut to determining the units of rate constant. [b] is the molar concentrations of. K(t) is the reaction rate constant that depends on temperature. Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the. K = rate/[a] x [b] y. The rate constant k and the. The Rate Constant K Is Given By.
From www.sliderbase.com
Determining Order with Concentration vs. Time data The Rate Constant K Is Given By Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant k and the exponents m, n, and p must. The Rate Constant K Is Given By.
From www.chegg.com
Solved Rate=k[A]⁴[B][C]² Using your rate law, calculate the The Rate Constant K Is Given By The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a. The Rate Constant K Is Given By.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q The Rate Constant K Is Given By K = rate/[a] x [b] y. K(t) is the reaction rate constant that depends on temperature. A shortcut to determining the units of rate constant. Therefore, the units of k (assuming that. Rearranging the rate equation, the value of the rate constant ‘k’ is given by: Notice how for each order, we determined the units of k by dividing the. The Rate Constant K Is Given By.
From www.toppr.com
The rate constant of a certain reaction is given by log10K = 5.3192 The Rate Constant K Is Given By K = rate/[a] x [b] y. The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: A shortcut to determining the units of rate constant. [b] is the molar concentrations of. Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the. The Rate Constant K Is Given By.
From www.chegg.com
Solved For a secondorder reaction, the rate constant k is The Rate Constant K Is Given By The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. [a] is the molar concentrations of substances a in moles per unit volume of solution. K = rate/[a] x [b] y. Therefore, the units of k (assuming that. K(t) is the reaction rate constant that depends on temperature.. The Rate Constant K Is Given By.
From www.chegg.com
Solved The temperature dependence of the reaction rate The Rate Constant K Is Given By The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: [b] is the molar concentrations of. K(t) is the reaction rate constant that depends on temperature. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. K = rate/[a]. The Rate Constant K Is Given By.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free The Rate Constant K Is Given By A shortcut to determining the units of rate constant. Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the. [b] is the molar concentrations of. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. [a] is the molar. The Rate Constant K Is Given By.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical The Rate Constant K Is Given By The rate constant for the first order decomposition of h 2 o 2 is given by the following equation: [a] is the molar concentrations of substances a in moles per unit volume of solution. K(t) is the reaction rate constant that depends on temperature. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a. The Rate Constant K Is Given By.
From byjus.com
WHY RATE CONSTANT(K) IS INDEPENDENT OF INITIAL CONCENTRATION OF REACTENT? The Rate Constant K Is Given By K(t) is the reaction rate constant that depends on temperature. A shortcut to determining the units of rate constant. Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the. Rearranging the rate equation, the value of the rate constant ‘k’ is given by: The rate constant k. The Rate Constant K Is Given By.
From www.numerade.com
SOLVEDThe equation for the rate constant is k=Ae^EakT, A chemical The Rate Constant K Is Given By [b] is the molar concentrations of. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. A shortcut to determining the units of rate constant. Therefore, the units of k (assuming that. K = rate/[a] x [b] y. K(t) is the reaction rate constant that depends on temperature. The rate constant k. The Rate Constant K Is Given By.
From www.youtube.com
For a reaction, the values of rate constant k at two The Rate Constant K Is Given By A shortcut to determining the units of rate constant. K(t) is the reaction rate constant that depends on temperature. K = rate/[a] x [b] y. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. [a] is the molar concentrations of substances a in moles per unit volume of solution. [b] is. The Rate Constant K Is Given By.
From www.solvedlib.com
Calculate three values for the rate constant; k, at r… SolvedLib The Rate Constant K Is Given By L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. A shortcut to determining the units of rate constant. Rearranging the rate equation, the value of the. The Rate Constant K Is Given By.
From www.numerade.com
SOLVED Which one of the following given graphs represents the The Rate Constant K Is Given By K(t) is the reaction rate constant that depends on temperature. K = rate/[a] x [b] y. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. [b] is the molar concentrations of. Rearranging the rate equation, the value of the rate constant ‘k’ is given by: L o. The Rate Constant K Is Given By.
From www.youtube.com
The rate constant `(K\')` of one reaction is double of the rate The Rate Constant K Is Given By [b] is the molar concentrations of. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. K(t) is the reaction rate constant that depends on temperature. A shortcut to determining the units of rate constant. Rearranging the rate equation, the value of the rate constant ‘k’ is given. The Rate Constant K Is Given By.
From www.chegg.com
Solved For a secondorder reaction, the rate constant k is The Rate Constant K Is Given By K = rate/[a] x [b] y. K(t) is the reaction rate constant that depends on temperature. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. A shortcut to determining the units of rate constant. L o g k = 14.2 − 1.0 × 10. The Rate Constant K Is Given By.
From www.doubtnut.com
Plots showing the variation of the rate constant (k) with temperature The Rate Constant K Is Given By [b] is the molar concentrations of. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. K = rate/[a] x [b] y. [a] is the molar concentrations of substances a in moles per unit volume of solution. Therefore, the units of k (assuming that. L o g k. The Rate Constant K Is Given By.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2054453 The Rate Constant K Is Given By Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. The rate constant k and the reaction orders m and n must be determined experimentally. The Rate Constant K Is Given By.
From www.chegg.com
Solved Define the terms rate constant k and half life The Rate Constant K Is Given By The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. Therefore, the units of k (assuming that. Notice how for each order, we determined the units of k by dividing. The Rate Constant K Is Given By.
From www.researchgate.net
Rate constant k (given as log 10 (k)) as a function of the inverse The Rate Constant K Is Given By Therefore, the units of k (assuming that. K(t) is the reaction rate constant that depends on temperature. K = rate/[a] x [b] y. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. [a] is the molar concentrations of substances a in moles per unit volume of solution.. The Rate Constant K Is Given By.
From www.slideshare.net
Chemical The Rate Constant K Is Given By [b] is the molar concentrations of. A shortcut to determining the units of rate constant. [a] is the molar concentrations of substances a in moles per unit volume of solution. L o g k = 14.2 − 1.0 × 10 4 k t calculate e a for this. Therefore, the units of k (assuming that. K = rate/[a] x [b]. The Rate Constant K Is Given By.
From www.bartleby.com
Answered The rate constant at 366 K for a… bartleby The Rate Constant K Is Given By Therefore, the units of k (assuming that. [a] is the molar concentrations of substances a in moles per unit volume of solution. Rearranging the rate equation, the value of the rate constant ‘k’ is given by: [b] is the molar concentrations of. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how. The Rate Constant K Is Given By.
From www.doubtnut.com
For a reaction, consider the plot of In K versus 1//T given in the fig The Rate Constant K Is Given By Notice how for each order, we determined the units of k by dividing the rate by molarity raised to the power of the. K = rate/[a] x [b] y. K(t) is the reaction rate constant that depends on temperature. [b] is the molar concentrations of. Therefore, the units of k (assuming that. A shortcut to determining the units of rate. The Rate Constant K Is Given By.
From askfilo.com
Plots showing the variation of the rate constant (k) with temperature (T).. The Rate Constant K Is Given By A shortcut to determining the units of rate constant. The rate constant k and the exponents m, n, and p must be determined experimentally by observing how the rate of a. K = rate/[a] x [b] y. [a] is the molar concentrations of substances a in moles per unit volume of solution. The rate constant k and the reaction orders. The Rate Constant K Is Given By.
From www.sliderbase.com
Determining Order with Concentration vs. Time data The Rate Constant K Is Given By The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Therefore, the units of k (assuming that. K = rate/[a] x [b] y. K(t) is the reaction rate constant that depends on temperature. The rate constant k and the exponents m, n, and p must. The Rate Constant K Is Given By.