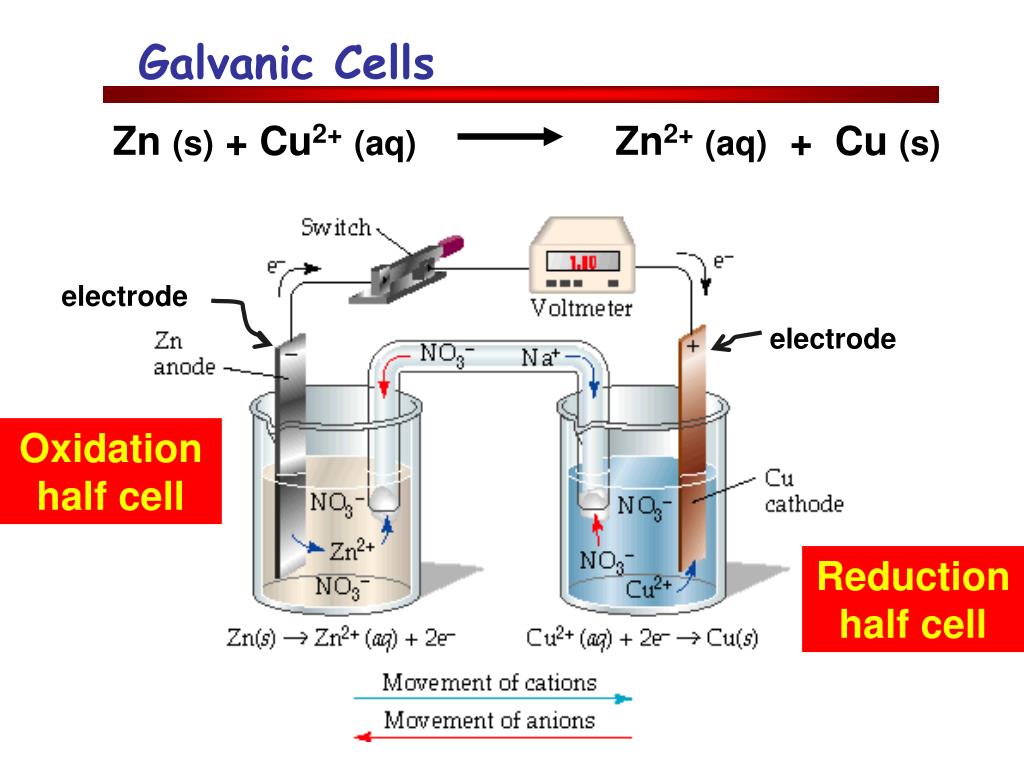
For A Galvanic Cell Zn/Zn2+ . (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. describe the function of a galvanic cell and its components; a galvanic cell contains two compartments: The picture opposite shows a galvanic cell made from zinc and tin half cells. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The zinc half cell has a strip. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. Use cell notation to symbolize the composition and. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper.
from www.slideserve.com
describe the function of a galvanic cell and its components; use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The picture opposite shows a galvanic cell made from zinc and tin half cells. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. Use cell notation to symbolize the composition and. The zinc half cell has a strip. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. a galvanic cell contains two compartments:
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902
For A Galvanic Cell Zn/Zn2+ The zinc half cell has a strip. The zinc half cell has a strip. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. describe the function of a galvanic cell and its components; To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. a galvanic cell contains two compartments: Use cell notation to symbolize the composition and. (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. The picture opposite shows a galvanic cell made from zinc and tin half cells.
From www.numerade.com
SOLVED What is the correct line notation for a galvanic cell composed For A Galvanic Cell Zn/Zn2+ in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. describe the function of a galvanic cell. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVEDINTERACTIVE EXAMPLE_Calculating AG for a Cell Reaction Consider For A Galvanic Cell Zn/Zn2+ use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The picture opposite shows a galvanic cell made from zinc and tin half cells. a galvanic cell contains two compartments: describe the function of a galvanic cell and its components; Use cell notation. For A Galvanic Cell Zn/Zn2+.
From www.toppr.com
Represent the galvanic cell in which the reaction takes place. Zn(s For A Galvanic Cell Zn/Zn2+ describe the function of a galvanic cell and its components; a galvanic cell contains two compartments: Use cell notation to symbolize the composition and. (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. The zinc half cell has a. For A Galvanic Cell Zn/Zn2+.
From byjus.com
For a galvanic cell Zn Zn2+ (C1) Zn2+(C2) Zn , E^° cell will be For A Galvanic Cell Zn/Zn2+ (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. use cell notation to describe the. For A Galvanic Cell Zn/Zn2+.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID1196366 For A Galvanic Cell Zn/Zn2+ Use cell notation to symbolize the composition and. The zinc half cell has a strip. The picture opposite shows a galvanic cell made from zinc and tin half cells. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. in a galvanic cell, current. For A Galvanic Cell Zn/Zn2+.
From www.toppr.com
Following reactions are taking place in a Galvanic cell, Zn→ Zn^2 For A Galvanic Cell Zn/Zn2+ a galvanic cell contains two compartments: in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. Use cell notation to symbolize the composition and. (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVEDIdentify the components of the galvanic electrochemical cell For A Galvanic Cell Zn/Zn2+ a galvanic cell contains two compartments: describe the function of a galvanic cell and its components; use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Use cell notation to symbolize the composition and. The picture opposite shows a galvanic cell made from. For A Galvanic Cell Zn/Zn2+.
From www.chegg.com
Solved The galvanic cell described by Zn(s) Zn2+ (aq) For A Galvanic Cell Zn/Zn2+ use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. The picture opposite shows a galvanic cell made. For A Galvanic Cell Zn/Zn2+.
From www.studyxapp.com
8 given zn2 2e zn cu 2e cu 076 v e034 v a sketch the galvanic cell For A Galvanic Cell Zn/Zn2+ The picture opposite shows a galvanic cell made from zinc and tin half cells. (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. Use cell notation to symbolize the composition and. a galvanic cell contains two compartments: use cell. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED Consider the galvanic cell with the following line notation Zn For A Galvanic Cell Zn/Zn2+ (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. a galvanic cell contains two compartments: To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper.. For A Galvanic Cell Zn/Zn2+.
From www.chegg.com
Solved A Zn(s) Zn2+(aq) Co2+(aq) Co(s) galvanic cell For A Galvanic Cell Zn/Zn2+ Use cell notation to symbolize the composition and. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions.. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED Question 37 1 pts For the galvanic cell Zn(s) 2TI (aq) 2TI(s For A Galvanic Cell Zn/Zn2+ The picture opposite shows a galvanic cell made from zinc and tin half cells. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
What is the potential difference (Ecell) in the galvanic cell shown For A Galvanic Cell Zn/Zn2+ The picture opposite shows a galvanic cell made from zinc and tin half cells. a galvanic cell contains two compartments: (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. The zinc half cell has a strip. describe the function. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED A Zn(s) Zn2+(aq) Co2+(aq) Co(s) galvanic cell has a For A Galvanic Cell Zn/Zn2+ Use cell notation to symbolize the composition and. The zinc half cell has a strip. The picture opposite shows a galvanic cell made from zinc and tin half cells. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. (a) a galvanic. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED A galvanic cell uses the reaction (Be) + Zn(s) → Zn2+(aq For A Galvanic Cell Zn/Zn2+ Use cell notation to symbolize the composition and. a galvanic cell contains two compartments: (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED For the galvanic cell reaction represented below, what half For A Galvanic Cell Zn/Zn2+ (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. a galvanic cell contains two compartments: The zinc half cell has a strip. Use cell notation to symbolize the composition and. use cell notation to describe the galvanic cell where. For A Galvanic Cell Zn/Zn2+.
From askfilo.com
24. The emf of a galvanic cell constituted l with the electrodes Zn2+/Zn⋅.. For A Galvanic Cell Zn/Zn2+ use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The picture opposite shows a galvanic cell made from zinc and tin half cells. The zinc half cell has a strip. a galvanic cell contains two compartments: Use cell notation to symbolize the composition. For A Galvanic Cell Zn/Zn2+.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID1196366 For A Galvanic Cell Zn/Zn2+ Use cell notation to symbolize the composition and. a galvanic cell contains two compartments: use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. describe the function of a galvanic cell and its components; in a galvanic cell, current is produced when. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED Question 3.b of 12 Consider a galvanic electrochemical cell For A Galvanic Cell Zn/Zn2+ (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. a galvanic cell contains two compartments: in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference. For A Galvanic Cell Zn/Zn2+.
From askfilo.com
Ans.(3) 17 A galvanic cell is constructed with Zn2+/Zn(E0=−0.76 V) and Fe.. For A Galvanic Cell Zn/Zn2+ (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Use cell notation to symbolize the composition. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED A galvanic cell is constructed with a Sn/Sn2+ halfcell and Zn For A Galvanic Cell Zn/Zn2+ The picture opposite shows a galvanic cell made from zinc and tin half cells. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. describe the function of a galvanic cell and its components; Use cell notation to symbolize the composition and. (a). For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVEDA galvanic cell is constructed from a Zn / Zn^2+ halfcell For A Galvanic Cell Zn/Zn2+ To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. describe the function of a galvanic cell and its components; (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of. For A Galvanic Cell Zn/Zn2+.
From www.toppr.com
Depict the galvanic cell in which the reaction Zn(s) + 2Ag^+(aq)→ Zn^2 For A Galvanic Cell Zn/Zn2+ The zinc half cell has a strip. The picture opposite shows a galvanic cell made from zinc and tin half cells. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. Use cell notation to symbolize the composition and. describe the function of a. For A Galvanic Cell Zn/Zn2+.
From www.solutionspile.com
[Solved] A galvanic cell using Cu+/Cu and Zn2+/Zn was set For A Galvanic Cell Zn/Zn2+ a galvanic cell contains two compartments: in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. . For A Galvanic Cell Zn/Zn2+.
From quizlet.com
Label the zinc/copper galvanic cell Diagram Quizlet For A Galvanic Cell Zn/Zn2+ use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. a galvanic cell contains two compartments: in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. . For A Galvanic Cell Zn/Zn2+.
From www.slideserve.com
PPT Making Galvanic Cells Lab PowerPoint Presentation, free download For A Galvanic Cell Zn/Zn2+ To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. a galvanic cell contains two compartments: describe the function of a galvanic cell and its components; The picture opposite shows a galvanic cell made from zinc and tin half cells. The zinc half. For A Galvanic Cell Zn/Zn2+.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 For A Galvanic Cell Zn/Zn2+ Use cell notation to symbolize the composition and. describe the function of a galvanic cell and its components; The picture opposite shows a galvanic cell made from zinc and tin half cells. The zinc half cell has a strip. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the. For A Galvanic Cell Zn/Zn2+.
From exowsidwq.blob.core.windows.net
Zinc How To Find Charge at Patel blog For A Galvanic Cell Zn/Zn2+ The zinc half cell has a strip. The picture opposite shows a galvanic cell made from zinc and tin half cells. in a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential. use cell notation to describe the galvanic cell where copper(ii) ions. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED Imagine a galvanic cell which uses solid zinc and aqueous iron For A Galvanic Cell Zn/Zn2+ The zinc half cell has a strip. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Use cell notation to symbolize the composition and. The picture opposite shows a galvanic cell made from zinc and tin half cells. a galvanic cell contains two. For A Galvanic Cell Zn/Zn2+.
From www.chegg.com
Solved A galvanic cell Zn∣∣Zn2+∥Ni2+∣∣ Ni runs For A Galvanic Cell Zn/Zn2+ To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper. The zinc half cell has a strip. (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions.. For A Galvanic Cell Zn/Zn2+.
From askfilo.com
JAC 5. A galvanic cell is constructed with Zn2+/Zn(E∘=−0.76 V) Cu2+/Cu(E∘.. For A Galvanic Cell Zn/Zn2+ describe the function of a galvanic cell and its components; use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of. For A Galvanic Cell Zn/Zn2+.
From www.chegg.com
Solved A galvanic cell using Au+3/Au and Zn2+ / Zn was set For A Galvanic Cell Zn/Zn2+ (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. a galvanic cell contains two compartments: The picture opposite shows a galvanic cell made from zinc and tin half cells. The zinc half cell has a strip. in a galvanic. For A Galvanic Cell Zn/Zn2+.
From www.slideserve.com
PPT The End is in Site! PowerPoint Presentation, free download ID For A Galvanic Cell Zn/Zn2+ use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. describe the function of a galvanic cell and its components; To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED The reactions for galvanic cell are as follows; Anode(oxidation For A Galvanic Cell Zn/Zn2+ a galvanic cell contains two compartments: use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The zinc half cell has a strip. Use cell notation to symbolize the composition and. The picture opposite shows a galvanic cell made from zinc and tin half. For A Galvanic Cell Zn/Zn2+.
From www.numerade.com
SOLVED Question 6 Volcmeter IM Zn"+ IM Cozr The figure shows schematic For A Galvanic Cell Zn/Zn2+ The zinc half cell has a strip. describe the function of a galvanic cell and its components; (a) a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 + ions. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of. For A Galvanic Cell Zn/Zn2+.