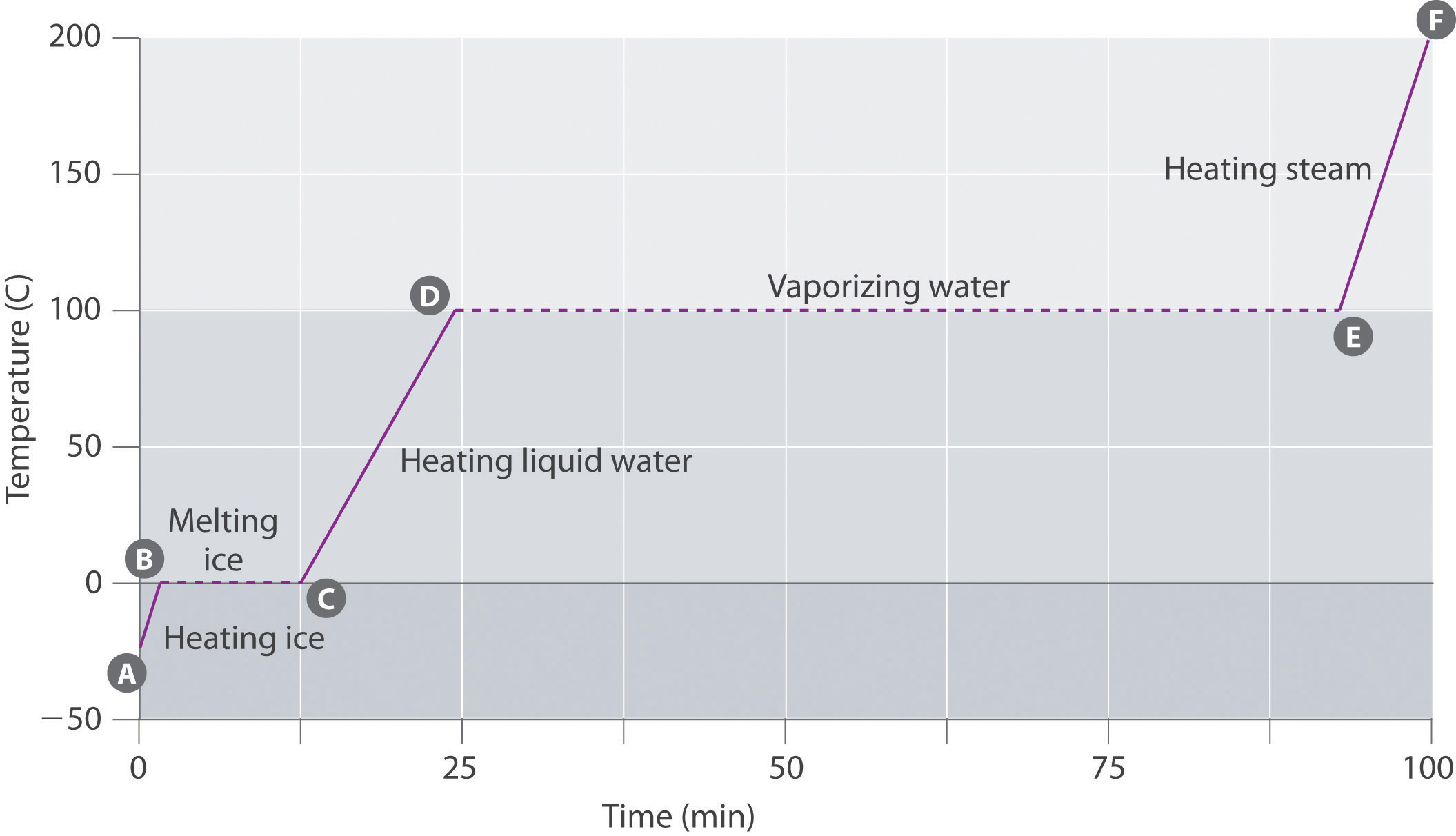
Distilled Water Boiling Temp . When it reaches t 1, we will have boiled away half of the liquid. As the liquid continues to boil, the boiling temperature rises. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘. However, you can obtain distilled water from damp soil, plants, snow, and rain, too. Doing this produces water that is free from minerals and other impurities. Distilled water is water purified by condensing water vapor into liquid water. A compound must satisfy three conditions to be successfully separated by steam distillation. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. As a result, the vapor. It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330 mmhg. If two or more compounds satisfy these three conditions, they will generally not separate. When the difference in boiling.
from chem.libretexts.org
If two or more compounds satisfy these three conditions, they will generally not separate. You can distill water to make drinking water for. When the difference in boiling. A simple distillation is incapable of significant purification if the boiling points of the components are too close. It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. When it reaches t 1, we will have boiled away half of the liquid. At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330 mmhg. A compound must satisfy three conditions to be successfully separated by steam distillation. Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. However, you can obtain distilled water from damp soil, plants, snow, and rain, too.
12.7 Heating Curve for Water Chemistry LibreTexts
Distilled Water Boiling Temp Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. As the liquid continues to boil, the boiling temperature rises. Doing this produces water that is free from minerals and other impurities. When it reaches t 1, we will have boiled away half of the liquid. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘. Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. However, you can obtain distilled water from damp soil, plants, snow, and rain, too. As a result, the vapor. At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330 mmhg. Distilled water is water purified by condensing water vapor into liquid water. A simple distillation is incapable of significant purification if the boiling points of the components are too close. It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. If two or more compounds satisfy these three conditions, they will generally not separate. When the difference in boiling. You can distill water to make drinking water for. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG Distilled Water Boiling Temp If two or more compounds satisfy these three conditions, they will generally not separate. You can distill water to make drinking water for. As a result, the vapor. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Usually, the distillation process involves boiling impure water and collected the condensed vapor in. Distilled Water Boiling Temp.
From www.perkinselearning.org
Can the Boiling Point of Water be Changed? Perkins eLearning Distilled Water Boiling Temp When the difference in boiling. Distilled water is water purified by condensing water vapor into liquid water. It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. You can distill water to make drinking water for. Usually, the distillation process. Distilled Water Boiling Temp.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Distilled Water Boiling Temp It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. Distilled water is water that has been boiled into steam, or evaporated into. Distilled Water Boiling Temp.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID Distilled Water Boiling Temp A simple distillation is incapable of significant purification if the boiling points of the components are too close. Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4. Distilled Water Boiling Temp.
From jsmithmoore.com
Boiling point of ethanol celsius Distilled Water Boiling Temp As a result, the vapor. You can distill water to make drinking water for. When the difference in boiling. It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. A simple distillation is incapable of significant purification if the boiling. Distilled Water Boiling Temp.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Distilled Water Boiling Temp Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. Distilled water is water purified by condensing water vapor into liquid water. If two or more compounds satisfy these three conditions, they will generally not separate. However, you can obtain distilled water from damp soil, plants, snow, and rain,. Distilled Water Boiling Temp.
From www.youtube.com
How to Make Double Distilled Water Distillation unit for distilled Distilled Water Boiling Temp When it reaches t 1, we will have boiled away half of the liquid. A simple distillation is incapable of significant purification if the boiling points of the components are too close. As a result, the vapor. You can distill water to make drinking water for. However, you can obtain distilled water from damp soil, plants, snow, and rain, too.. Distilled Water Boiling Temp.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Tessshebaylo Distilled Water Boiling Temp However, you can obtain distilled water from damp soil, plants, snow, and rain, too. Doing this produces water that is free from minerals and other impurities. You can distill water to make drinking water for. When it reaches t 1, we will have boiled away half of the liquid. If two or more compounds satisfy these three conditions, they will. Distilled Water Boiling Temp.
From microbenotes.com
Water Distiller Principle, Parts, Types, Uses, Examples Distilled Water Boiling Temp At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330 mmhg. As the liquid continues to boil, the boiling temperature rises. However, you can obtain distilled water from damp soil, plants, snow, and rain, too. Usually, the distillation process involves boiling impure water and collected the condensed vapor. Distilled Water Boiling Temp.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Point Tessshebaylo Distilled Water Boiling Temp Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. Distilled water is water purified by condensing water vapor into liquid water. You can distill water to make drinking water for. Doing this produces water that is free from minerals and other impurities. It must be stable and relatively insoluble in boiling water,. Distilled Water Boiling Temp.
From waterfilterguru.com
How to Make Distilled Water in 5 Easy Steps Distilled Water Boiling Temp However, you can obtain distilled water from damp soil, plants, snow, and rain, too. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. As the liquid continues to boil, the boiling temperature rises. A simple distillation is incapable of significant purification if the boiling points of the components are too close. At. Distilled Water Boiling Temp.
From www.britannica.com
distillation summary Britannica Distilled Water Boiling Temp If two or more compounds satisfy these three conditions, they will generally not separate. Distilled water is water purified by condensing water vapor into liquid water. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘. Usually, the distillation process involves boiling impure water and collected the. Distilled Water Boiling Temp.
From sciencenotes.org
How to Boil Water at Room Temperature Distilled Water Boiling Temp At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330 mmhg. A compound must satisfy three conditions to be successfully separated by steam distillation. Distilled water is water purified by condensing water vapor into liquid water. If two or more compounds satisfy these three conditions, they will generally. Distilled Water Boiling Temp.
From www.scribd.com
Preparation of Distilled Water PDF Distillation Boiling Distilled Water Boiling Temp As a result, the vapor. At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330 mmhg. A compound must satisfy three conditions to be successfully separated by steam distillation. However, you can obtain distilled water from damp soil, plants, snow, and rain, too. Doing this produces water that. Distilled Water Boiling Temp.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Point Tessshebaylo Distilled Water Boiling Temp As the liquid continues to boil, the boiling temperature rises. When the difference in boiling. If two or more compounds satisfy these three conditions, they will generally not separate. It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. You. Distilled Water Boiling Temp.
From chem.libretexts.org
12.7 Heating Curve for Water Chemistry LibreTexts Distilled Water Boiling Temp A simple distillation is incapable of significant purification if the boiling points of the components are too close. When the difference in boiling. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. A compound must satisfy three conditions to be successfully separated by steam distillation. Doing this produces water that is free. Distilled Water Boiling Temp.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG Distilled Water Boiling Temp However, you can obtain distilled water from damp soil, plants, snow, and rain, too. Distilled water is water purified by condensing water vapor into liquid water. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. If two or more compounds satisfy these three conditions, they will generally not separate. A simple distillation. Distilled Water Boiling Temp.
From exyftyaah.blob.core.windows.net
Distilled Water Boiling Point Fahrenheit at Eugene Lee blog Distilled Water Boiling Temp However, you can obtain distilled water from damp soil, plants, snow, and rain, too. Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. Doing this produces water that is free from minerals and other impurities. When the difference in boiling. As a result, the vapor. It must be. Distilled Water Boiling Temp.
From sciencenotes.org
How to Boil Water at Room Temperature Distilled Water Boiling Temp However, you can obtain distilled water from damp soil, plants, snow, and rain, too. When it reaches t 1, we will have boiled away half of the liquid. As the liquid continues to boil, the boiling temperature rises. At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330. Distilled Water Boiling Temp.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Distilled Water Boiling Temp Doing this produces water that is free from minerals and other impurities. Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1. Distilled Water Boiling Temp.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level Distilled Water Boiling Temp If two or more compounds satisfy these three conditions, they will generally not separate. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘. It must be stable and. Distilled Water Boiling Temp.
From lisassimplelife.com
Distilled waterBoiling Process for Distilled Water Lisa's Simple Life Distilled Water Boiling Temp If two or more compounds satisfy these three conditions, they will generally not separate. At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330 mmhg. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. You can distill water to make. Distilled Water Boiling Temp.
From www.youtube.com
how to prepare distilled water distilled water preparation in Distilled Water Boiling Temp It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. A simple distillation is incapable of significant purification if the boiling points of the components are too close. If two or more compounds satisfy these three conditions, they will generally. Distilled Water Boiling Temp.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Distilled Water Boiling Temp When the difference in boiling. Doing this produces water that is free from minerals and other impurities. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘. You can. Distilled Water Boiling Temp.
From mavink.com
Elevation In Boiling Point Graph Distilled Water Boiling Temp At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330 mmhg. Doing this produces water that is free from minerals and other impurities. It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order. Distilled Water Boiling Temp.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free Distilled Water Boiling Temp You can distill water to make drinking water for. It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4. Distilled Water Boiling Temp.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Distilled Water Boiling Temp A simple distillation is incapable of significant purification if the boiling points of the components are too close. Doing this produces water that is free from minerals and other impurities. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. When it reaches t 1, we will have boiled away half of the. Distilled Water Boiling Temp.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning Distilled Water Boiling Temp As a result, the vapor. However, you can obtain distilled water from damp soil, plants, snow, and rain, too. If two or more compounds satisfy these three conditions, they will generally not separate. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Distilled water is water purified by condensing water vapor. Distilled Water Boiling Temp.
From www.thoughtco.com
How to Make Distilled Water Distilled Water Boiling Temp Doing this produces water that is free from minerals and other impurities. As the liquid continues to boil, the boiling temperature rises. Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while. Distilled Water Boiling Temp.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Distilled Water Boiling Temp When the difference in boiling. Doing this produces water that is free from minerals and other impurities. A simple distillation is incapable of significant purification if the boiling points of the components are too close. Distilled water is water purified by condensing water vapor into liquid water. It must be stable and relatively insoluble in boiling water, and it must. Distilled Water Boiling Temp.
From microbenotes.com
Water Distiller Principle, Parts, Types, Uses, Examples Distilled Water Boiling Temp Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘. It must be stable and relatively insoluble in boiling water, and it must have a vapor. Distilled Water Boiling Temp.
From www.researchgate.net
Temperature variation for water boiling test Download Scientific Diagram Distilled Water Boiling Temp It must be stable and relatively insoluble in boiling water, and it must have a vapor pressure in boiling water that is of the order of 1 kpa (0.01) atmosphere. A compound must satisfy three conditions to be successfully separated by steam distillation. A simple distillation is incapable of significant purification if the boiling points of the components are too. Distilled Water Boiling Temp.
From worldnewlive.com
At What Temperature Did The Water Start To Boil? Mastery Wiki Distilled Water Boiling Temp Doing this produces water that is free from minerals and other impurities. Distilled water is water that has been boiled into steam, or evaporated into vapor, and then recondensed into a different container. You can distill water to make drinking water for. Usually, the distillation process involves boiling impure water and collected the condensed vapor in a fresh container. When. Distilled Water Boiling Temp.
From chem.libretexts.org
10.5 Water Properties Chemistry LibreTexts Distilled Water Boiling Temp Simple distillation in the lab is generally appropriate for separating out a molecular substance with a boiling point between ∼ 4 0 ∘. Distilled water is water purified by condensing water vapor into liquid water. You can distill water to make drinking water for. When the difference in boiling. If two or more compounds satisfy these three conditions, they will. Distilled Water Boiling Temp.
From georgeperestheoxiia4.blogspot.com
Diy Distilled Water Machine 3 Ways To Make Distilled Water Wikihow Distilled Water Boiling Temp At the normal boiling point of ethanol, the vapor pressure of pure ethanol is 760 mmhg, while that of water is ~330 mmhg. Doing this produces water that is free from minerals and other impurities. However, you can obtain distilled water from damp soil, plants, snow, and rain, too. It must be stable and relatively insoluble in boiling water, and. Distilled Water Boiling Temp.