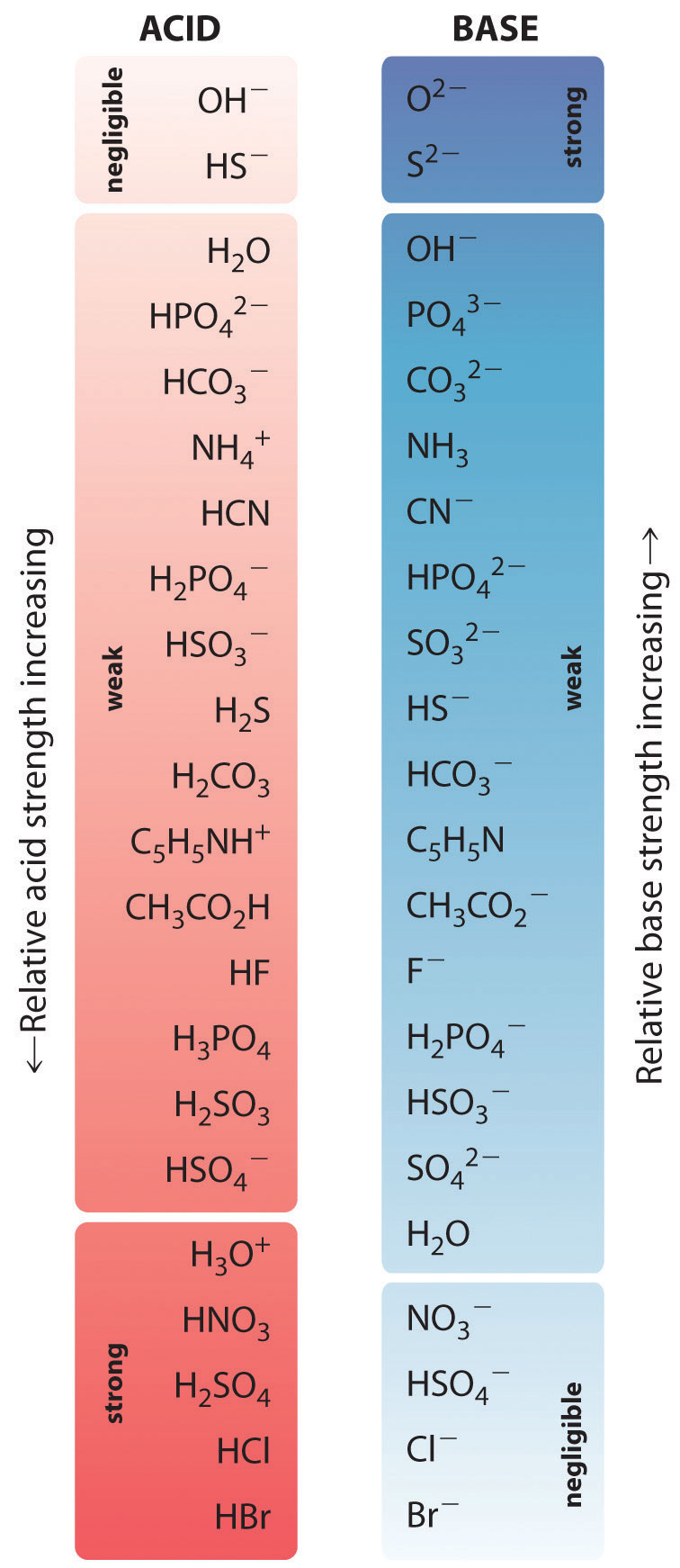
Exceptionally Low Acidic Strength Of Hf Is Due To . The partially positive hydrogen is trapped between two highly electronegative atoms. The charge, the atom, resonance, inductive effects, and the orbitals. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. We assess the extent of the following. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. Hydrogen bonding in hf is the reason for its low acidic strength. There are five key factors that influence acidity in organic chemistry; Because of the fluoride ion's. Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. This page discusses the acidity of the hydrogen halides: Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides. The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base.
from architecturalstudio.com
There are five key factors that influence acidity in organic chemistry; Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. The charge, the atom, resonance, inductive effects, and the orbitals. This page discusses the acidity of the hydrogen halides: Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. Because of the fluoride ion's. Hydrogen bonding in hf is the reason for its low acidic strength. The partially positive hydrogen is trapped between two highly electronegative atoms. We assess the extent of the following. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,.
Acid Strengths Table
Exceptionally Low Acidic Strength Of Hf Is Due To We assess the extent of the following. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. We assess the extent of the following. Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. Because of the fluoride ion's. There are five key factors that influence acidity in organic chemistry; This page discusses the acidity of the hydrogen halides: The charge, the atom, resonance, inductive effects, and the orbitals. Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Hydrogen bonding in hf is the reason for its low acidic strength. The partially positive hydrogen is trapped between two highly electronegative atoms.
From www.w3schools.blog
Acid strength W3schools Exceptionally Low Acidic Strength Of Hf Is Due To This page discusses the acidity of the hydrogen halides: Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides.. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.doubtnut.com
Write correct order of acidic strength of following compounds Exceptionally Low Acidic Strength Of Hf Is Due To We assess the extent of the following. Hydrogen bonding in hf is the reason for its low acidic strength. The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. There are five key factors that influence acidity in organic chemistry; The partially positive hydrogen. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.esaral.com
Acidic Strength and Basic Strength Revision Class 11, JEE, NEET Exceptionally Low Acidic Strength Of Hf Is Due To Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides. Hydrogen bonding in hf is the reason for its low acidic strength. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.thoughtco.com
List of Common Strong and Weak Acids Exceptionally Low Acidic Strength Of Hf Is Due To Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. This page discusses the acidity of the hydrogen halides: The partially positive hydrogen is trapped between two highly electronegative atoms. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Hydrofluoric acid is the. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.flinnsci.ca
AcidBase Strength Charts for Chemistry Exceptionally Low Acidic Strength Of Hf Is Due To Hydrogen bonding in hf is the reason for its low acidic strength. Because of the fluoride ion's. The charge, the atom, resonance, inductive effects, and the orbitals. There are five key factors that influence acidity in organic chemistry; Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.doubtnut.com
What is the increasing order of acidic strength of the following acid Exceptionally Low Acidic Strength Of Hf Is Due To There are five key factors that influence acidity in organic chemistry; Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. The partially positive hydrogen is trapped. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.meritnation.com
Correct order of acidic strength Chemistry Alcohols Phenols and Exceptionally Low Acidic Strength Of Hf Is Due To Because of the fluoride ion's. The charge, the atom, resonance, inductive effects, and the orbitals. This page discusses the acidity of the hydrogen halides: We assess the extent of the following. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Hydrofluoric acid. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.youtube.com
Why hydrogen fluoride is a weaker acid than hydrochloric acid Find Exceptionally Low Acidic Strength Of Hf Is Due To Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. The partially positive hydrogen is trapped between two highly electronegative atoms. This page discusses the acidity of the hydrogen halides: Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. The. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.doubtnut.com
Write correct order of acidic strength of following compounds Exceptionally Low Acidic Strength Of Hf Is Due To Because of the fluoride ion's. There are five key factors that influence acidity in organic chemistry; Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. We assess the extent of. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.youtube.com
acidic strength (all effects) YouTube Exceptionally Low Acidic Strength Of Hf Is Due To Hydrogen bonding in hf is the reason for its low acidic strength. There are five key factors that influence acidity in organic chemistry; Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. The partially positive hydrogen is trapped between two highly electronegative atoms. Because of the fluoride ion's. The increase in acid strength with increasing number of. Exceptionally Low Acidic Strength Of Hf Is Due To.
From testbook.com
Acidic Strength Learn Definition, Strength of Strong & Weak Acid Exceptionally Low Acidic Strength Of Hf Is Due To Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides. We assess the extent of the following. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. This page discusses the acidity of the hydrogen halides: The partially positive hydrogen is trapped between two highly electronegative atoms. Because of the fluoride ion's. Since both entropy. Exceptionally Low Acidic Strength Of Hf Is Due To.
From mavink.com
Acid Base Strength Chart Exceptionally Low Acidic Strength Of Hf Is Due To There are five key factors that influence acidity in organic chemistry; The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.gkseries.com
In terms of acidic strength, which one of the following is in the Exceptionally Low Acidic Strength Of Hf Is Due To The charge, the atom, resonance, inductive effects, and the orbitals. This page discusses the acidity of the hydrogen halides: There are five key factors that influence acidity in organic chemistry; The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. We assess the extent. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.toppr.com
Correct order of Acidic strength Exceptionally Low Acidic Strength Of Hf Is Due To There are five key factors that influence acidity in organic chemistry; Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides. The partially positive hydrogen is trapped between two highly electronegative atoms. We assess the extent of the following. Because of the fluoride ion's. Hydrogen bonding in hf is the reason for its low. Exceptionally Low Acidic Strength Of Hf Is Due To.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry Atoms First Exceptionally Low Acidic Strength Of Hf Is Due To Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. Hydrogen bonding in hf is the reason for its low acidic strength.. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 Exceptionally Low Acidic Strength Of Hf Is Due To We assess the extent of the following. This page discusses the acidity of the hydrogen halides: Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides. The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. The charge,. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.youtube.com
What is the Order of Relative Acid Strength of Halogen acids, HF, HCl Exceptionally Low Acidic Strength Of Hf Is Due To Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. The charge, the atom, resonance, inductive effects, and the orbitals. Both entropy and enthalpy reduce the acidity of hf with respect. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.vrogue.co
Order Of Acidic Strength Chemistry Questions vrogue.co Exceptionally Low Acidic Strength Of Hf Is Due To We assess the extent of the following. Hydrogen bonding in hf is the reason for its low acidic strength. This page discusses the acidity of the hydrogen halides: Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. There are five key factors that influence acidity in organic chemistry; Because of the fluoride ion's. The increase in acid. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.doubtnut.com
The correct order of acidic strength is Exceptionally Low Acidic Strength Of Hf Is Due To Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. Hydrogen bonding in hf is the reason for its low acidic strength. We assess the extent of the following. Because of the fluoride ion's. There. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.masterorganicchemistry.com
5 Key Factors That Influence Acidity In Organic Chemistry Exceptionally Low Acidic Strength Of Hf Is Due To Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. The charge, the atom, resonance, inductive effects, and the orbitals. There are five key factors that influence acidity in organic chemistry; Hydrofluoric acid is. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.numerade.com
SOLVED 'pls answer this question Which of the following trend is Exceptionally Low Acidic Strength Of Hf Is Due To The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. We assess the extent of the following. There are five key factors that influence acidity in organic chemistry; Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. Since both entropy and. Exceptionally Low Acidic Strength Of Hf Is Due To.
From architecturalstudio.com
Acid Strengths Table Exceptionally Low Acidic Strength Of Hf Is Due To Because of the fluoride ion's. Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides. Hydrogen bonding in hf is the reason for its low acidic strength. We assess the extent of the following. The partially positive hydrogen is trapped between two highly electronegative atoms. This page discusses the acidity of the hydrogen halides:. Exceptionally Low Acidic Strength Of Hf Is Due To.
From ar.inspiredpencil.com
Intermolecular Forces Hf Exceptionally Low Acidic Strength Of Hf Is Due To Because of the fluoride ion's. We assess the extent of the following. This page discusses the acidity of the hydrogen halides: Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Both entropy and. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.youtube.com
Factors affecting acid strength Acids and bases AP Chemistry Khan Exceptionally Low Acidic Strength Of Hf Is Due To Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. There are five key factors that influence acidity in organic chemistry; This page discusses the acidity of the hydrogen halides: The partially positive hydrogen is trapped between two highly electronegative atoms. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. Since both entropy and enthalpy is disfavoured with. Exceptionally Low Acidic Strength Of Hf Is Due To.
From scoop.eduncle.com
Identify correct acidic strength order in the following compounds ho Exceptionally Low Acidic Strength Of Hf Is Due To This page discusses the acidity of the hydrogen halides: Because of the fluoride ion's. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. Since both entropy and enthalpy is disfavoured with respect to fluoride,. Exceptionally Low Acidic Strength Of Hf Is Due To.
From askfilo.com
What is the order of acidic strength of the labelled H atoms Filo Exceptionally Low Acidic Strength Of Hf Is Due To The partially positive hydrogen is trapped between two highly electronegative atoms. We assess the extent of the following. There are five key factors that influence acidity in organic chemistry; Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. The charge, the atom,. Exceptionally Low Acidic Strength Of Hf Is Due To.
From byjus.com
Statement I Acid strength increases in the order given as HF Exceptionally Low Acidic Strength Of Hf Is Due To Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Hydrogen bonding in hf is the reason for its low acidic strength. The partially positive hydrogen is trapped between two highly electronegative atoms. Hydrofluoric. Exceptionally Low Acidic Strength Of Hf Is Due To.
From askfilo.com
97. Determine the correct order of acidic strength) Filo Exceptionally Low Acidic Strength Of Hf Is Due To We assess the extent of the following. Because of the fluoride ion's. Hydrogen bonding in hf is the reason for its low acidic strength. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide.. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.pearson.com
Using the table below, which is a stronger acid HPO42 or HF Exceptionally Low Acidic Strength Of Hf Is Due To Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. Both entropy and enthalpy reduce the acidity of hf with respect to the lower hydrogen halides. There are five key factors that influence acidity in organic chemistry; We assess the extent of the following. The increase in acid strength. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.youtube.com
Acid Acidic Strength Goc LECTURE14 IITJEE NEET GENERAL Exceptionally Low Acidic Strength Of Hf Is Due To The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1302194 Exceptionally Low Acidic Strength Of Hf Is Due To Hydrogen bonding in hf is the reason for its low acidic strength. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. There are five key factors that influence acidity in organic chemistry; The charge, the atom, resonance, inductive effects, and the orbitals.. Exceptionally Low Acidic Strength Of Hf Is Due To.
From edurev.in
How to find acidic strength order and basic strength order? EduRev Exceptionally Low Acidic Strength Of Hf Is Due To There are five key factors that influence acidity in organic chemistry; The charge, the atom, resonance, inductive effects, and the orbitals. The partially positive hydrogen is trapped between two highly electronegative atoms. The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. Because of. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.youtube.com
Arrange the following acids in the decreasing order of their acid Exceptionally Low Acidic Strength Of Hf Is Due To The partially positive hydrogen is trapped between two highly electronegative atoms. The charge, the atom, resonance, inductive effects, and the orbitals. Because of the fluoride ion's. The increase in acid strength with increasing number of terminal oxygen atoms is due to both an inductive effect and increased stabilization of the conjugate base. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. Exceptionally Low Acidic Strength Of Hf Is Due To.
From www.doubtnut.com
Write correct order of acidic strength of following compounds Exceptionally Low Acidic Strength Of Hf Is Due To Because of the fluoride ion's. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. We assess the extent of the following. This page discusses the acidity of the hydrogen halides: Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is. Exceptionally Low Acidic Strength Of Hf Is Due To.
From 88guru.com
Acid Strength I Strong and Weak Acids I Determining Factors Exceptionally Low Acidic Strength Of Hf Is Due To There are five key factors that influence acidity in organic chemistry; Since both entropy and enthalpy is disfavoured with respect to fluoride, the result is that h f is a poor acid compared to h cl, h br,. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. Hydrogen bonding in hf is the reason for its low acidic strength. The. Exceptionally Low Acidic Strength Of Hf Is Due To.