What Is Meant By Drug Bioavailability . Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. Bioavailability of a single drug may vary. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability is the term most often used to characterize drug absorption. This term has been defined as the relative amount of a drug. Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment.
from eduinput.com
Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. Bioavailability is the term most often used to characterize drug absorption. This term has been defined as the relative amount of a drug. Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability of a single drug may vary. Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action.
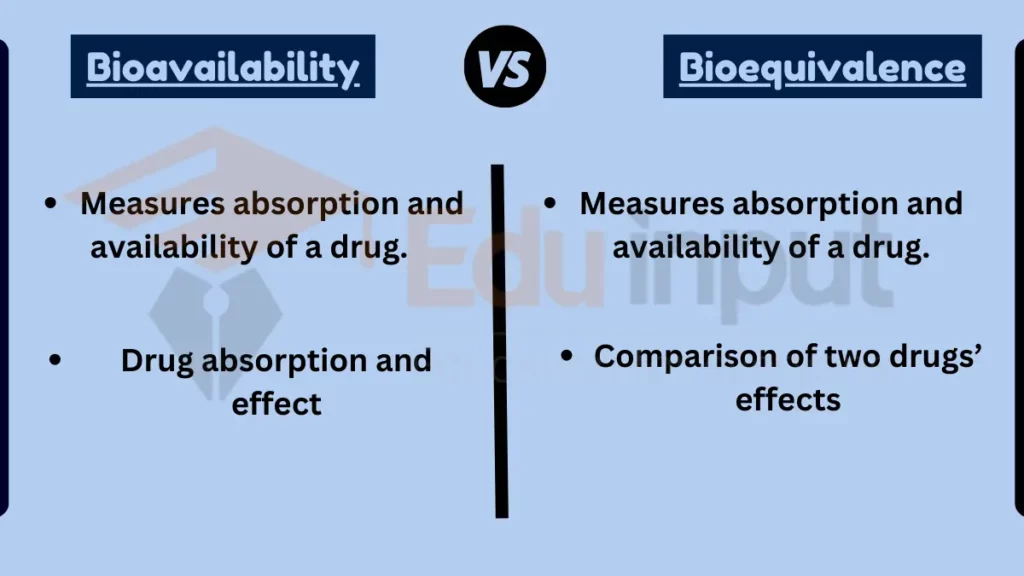
Difference Between Bioavailability and Bioequivalence
What Is Meant By Drug Bioavailability Bioavailability of a single drug may vary. Bioavailability is the term most often used to characterize drug absorption. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. This term has been defined as the relative amount of a drug. Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. Bioavailability of a single drug may vary.
From labbyag.es
Bioavailability Chart Labb by AG What Is Meant By Drug Bioavailability Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy.. What Is Meant By Drug Bioavailability.
From osmanplay.com
Basic Terms and Definition used in Bioavailability Curve and Factors What Is Meant By Drug Bioavailability Bioavailability of a single drug may vary. Bioavailability is the term most often used to characterize drug absorption. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug).. What Is Meant By Drug Bioavailability.
From tmedweb.tulane.edu
bioavailability_the_first_pass_effect [TUSOM Pharmwiki] What Is Meant By Drug Bioavailability Bioavailability is the term most often used to characterize drug absorption. Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability refers to the extent and rate at which the active moiety (drug or. What Is Meant By Drug Bioavailability.
From slidetodoc.com
GENERAL PHARMACOLOGY BIOAVAILABILITY BIOAVAILABILITY What Is Meant By Drug Bioavailability Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability of a. What Is Meant By Drug Bioavailability.
From www.researchgate.net
Most important factors affecting human oral bioavailability. Download What Is Meant By Drug Bioavailability Bioavailability of a single drug may vary. This term has been defined as the relative amount of a drug. Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. Bioavailability is the term most often used to characterize drug absorption. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend. What Is Meant By Drug Bioavailability.
From www.slideshare.net
Bioavailability studies lecture7 What Is Meant By Drug Bioavailability Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. Bioavailability of a single drug may vary. More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; The absolute bioavailability is ≥85% or when. What Is Meant By Drug Bioavailability.
From toolbox.eupati.eu
Bioavailability and bioequivalence EUPATI Toolbox What Is Meant By Drug Bioavailability More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. This term has been defined as the relative amount of a drug. Bioavailability refers to the. What Is Meant By Drug Bioavailability.
From www.slideshare.net
Bioavailability and Factors Affecting Bioavailability of drug What Is Meant By Drug Bioavailability Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. This term has been defined as the relative amount of a drug. Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. Bioavailability is the term. What Is Meant By Drug Bioavailability.
From www.slideserve.com
PPT Bioavailability PowerPoint Presentation, free download ID5176810 What Is Meant By Drug Bioavailability Bioavailability of a single drug may vary. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability is the term most often used to characterize drug absorption. Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. More accurately,. What Is Meant By Drug Bioavailability.
From swissrelief.com
What Does ‘bioavailability’ Mean? Swiss Relief What Is Meant By Drug Bioavailability More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; Bioavailability is the term most often used to characterize drug absorption. Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. Bioavailability refers to the extent and rate. What Is Meant By Drug Bioavailability.
From slidetodoc.com
GENERAL PHARMACOLOGY BIOAVAILABILITY BIOAVAILABILITY What Is Meant By Drug Bioavailability Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. Bioavailability of a single drug may vary. Bioavailability of a drug. What Is Meant By Drug Bioavailability.
From biologysimple.com
Bioavailability Biology Simple What Is Meant By Drug Bioavailability This term has been defined as the relative amount of a drug. Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability of a single drug may vary. Bioavailability. What Is Meant By Drug Bioavailability.
From pharmwarthegame.blogspot.com
Bioavailability What Is Meant By Drug Bioavailability Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. This term has. What Is Meant By Drug Bioavailability.
From www.slideserve.com
PPT Bioavailability PowerPoint Presentation, free download ID5176810 What Is Meant By Drug Bioavailability Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. Bioavailability is the term most often used to characterize drug absorption. Bioavailability (f) describes the fraction of a. What Is Meant By Drug Bioavailability.
From slideplayer.com
Bioavailability and Bioequivalence General concepts and overview ppt What Is Meant By Drug Bioavailability Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. This term has been defined as the relative amount of a drug. Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. Bioavailability refers to the extent and rate at which the active. What Is Meant By Drug Bioavailability.
From slidetodoc.com
GENERAL PHARMACOLOGY BIOAVAILABILITY BIOAVAILABILITY What Is Meant By Drug Bioavailability Bioavailability of a single drug may vary. Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). This term has been defined as the relative amount of a drug. Bioavailability. What Is Meant By Drug Bioavailability.
From www.slideserve.com
PPT Evolving Science in Bioavailability and Bioequivalence PowerPoint What Is Meant By Drug Bioavailability Bioavailability of a single drug may vary. More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; This term has been defined as the relative amount of a drug. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent. What Is Meant By Drug Bioavailability.
From www.slideshare.net
Bioavailability What Is Meant By Drug Bioavailability Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability of a single drug may vary. This term has been defined as the relative amount of a. What Is Meant By Drug Bioavailability.
From www.slideserve.com
PPT Week 6 Bioavailability and Bioequivalence PowerPoint What Is Meant By Drug Bioavailability Bioavailability of a single drug may vary. Bioavailability is the term most often used to characterize drug absorption. Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. This term has. What Is Meant By Drug Bioavailability.
From www.slideserve.com
PPT Bioavailability and Bioequivalence PowerPoint Presentation, free What Is Meant By Drug Bioavailability This term has been defined as the relative amount of a drug. Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability refers to the extent and rate at. What Is Meant By Drug Bioavailability.
From www.mdpi.com
Biomedicines Free FullText Bioavailability Enhancement Techniques What Is Meant By Drug Bioavailability Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. Bioavailability of a single drug may vary. Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action.. What Is Meant By Drug Bioavailability.
From eduinput.com
Difference Between Bioavailability and Bioequivalence What Is Meant By Drug Bioavailability Bioavailability is the term most often used to characterize drug absorption. Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully. What Is Meant By Drug Bioavailability.
From mungfali.com
Drug Bioavailability Chart What Is Meant By Drug Bioavailability Bioavailability is the term most often used to characterize drug absorption. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). This term has been defined as the relative amount of a drug. Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic. What Is Meant By Drug Bioavailability.
From ditki.com
Clinical Pharmacology Glossary Bioavailability ditki medical What Is Meant By Drug Bioavailability Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. Bioavailability of a single drug. What Is Meant By Drug Bioavailability.
From www.youtube.com
part 1 Overview, Absorption and Bioavailability What Is Meant By Drug Bioavailability Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure. What Is Meant By Drug Bioavailability.
From www.youtube.com
Bioavailability of Drugs Pharmacy Notes YouTube What Is Meant By Drug Bioavailability Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. This term has been. What Is Meant By Drug Bioavailability.
From www.yumpu.com
What Is the Bioavailability of Drugs What Is Meant By Drug Bioavailability Drug bioavailability is a crucial aspect of pharmacology, affecting the effectiveness of drug therapy. Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. This term has been defined as the relative amount. What Is Meant By Drug Bioavailability.
From mungfali.com
Drug Bioavailability Chart What Is Meant By Drug Bioavailability More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; Bioavailability of a single drug may vary. Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. This term has been defined as the relative amount of a drug. Bioavailability of a drug. What Is Meant By Drug Bioavailability.
From www.studypool.com
SOLUTION Pharmaceutical factors affecting drug bioavailability Studypool What Is Meant By Drug Bioavailability This term has been defined as the relative amount of a drug. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; Bioavailability (f) describes the fraction of. What Is Meant By Drug Bioavailability.
From www.slideserve.com
PPT Bioavailability & Absorption PowerPoint Presentation, free What Is Meant By Drug Bioavailability This term has been defined as the relative amount of a drug. Bioavailability of a single drug may vary. Bioavailability is the term most often used to characterize drug absorption. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. The absolute bioavailability is ≥85% or when ≥85%. What Is Meant By Drug Bioavailability.
From www.slideshare.net
Bioavailability and Factors Affecting Bioavailability of drug What Is Meant By Drug Bioavailability Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. This term has been defined as the relative amount of a drug. Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. Bioavailability is the term most often used to characterize. What Is Meant By Drug Bioavailability.
From www.studypool.com
SOLUTION Pharmaceutical factors affecting drug bioavailability Studypool What Is Meant By Drug Bioavailability Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). This term has been defined as the relative amount of a drug. Bioavailability is the term most often used to. What Is Meant By Drug Bioavailability.
From www.slideserve.com
PPT Bioavailability PowerPoint Presentation, free What Is Meant By Drug Bioavailability Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Understanding how drugs are absorbed, distributed, metabolized, and eliminated in patients’ bodies is essential to ensure proper and safe treatment. Bioavailability of a single drug. What Is Meant By Drug Bioavailability.
From www.pharmaexcipients.com
Advancement in Solubilization Approaches A Step towards What Is Meant By Drug Bioavailability Bioavailability of a single drug may vary. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. Bioavailability is the term most often used to characterize drug absorption. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug).. What Is Meant By Drug Bioavailability.
From www.slideserve.com
PPT Bioavailability PowerPoint Presentation, free download ID5176810 What Is Meant By Drug Bioavailability Bioavailability (f) describes the fraction of a dose of drug that reaches the systemic circulation. The absolute bioavailability is ≥85% or when ≥85% of the administered dose is recovered in urine as unchanged (parent drug). Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. This term has. What Is Meant By Drug Bioavailability.