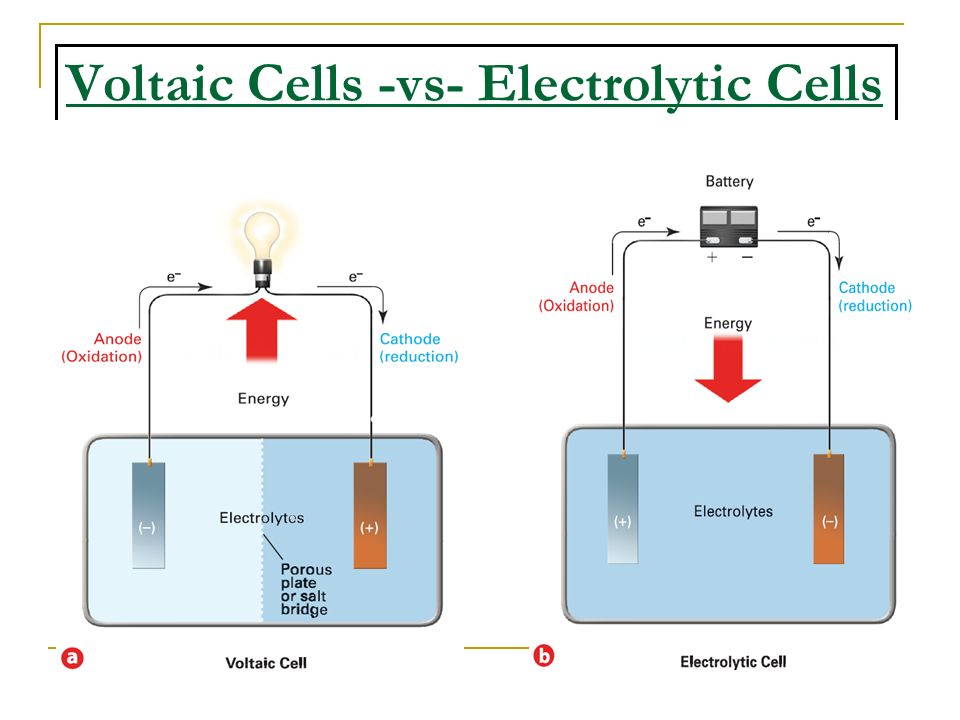
Electrochemical Cell Mcat . • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. Electrochemical cells have two conductive electrodes, called the anode and the cathode. On the diagram, this is represented by a battery in the circuit. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. We’ll discuss how these concepts are applied using different electrochemical cells. Below is a typical setup for an electrochemical cell! Within all electrochemical cells, there are 3 main components: In contrast, a galvanic cell has in its place either a. The anode is defined as the electrode where. In this article, we’ll go over everything you need to know about electrochemistry for the mcat. • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode.
from www.reddit.com
• the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. Electrochemical cells have two conductive electrodes, called the anode and the cathode. • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. The anode is defined as the electrode where. Within all electrochemical cells, there are 3 main components: Below is a typical setup for an electrochemical cell! On the diagram, this is represented by a battery in the circuit. In contrast, a galvanic cell has in its place either a. In this article, we’ll go over everything you need to know about electrochemistry for the mcat.
AAMC Physics QPack 27 Electron Movement r/Mcat
Electrochemical Cell Mcat • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. Below is a typical setup for an electrochemical cell! Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. In this article, we’ll go over everything you need to know about electrochemistry for the mcat. On the diagram, this is represented by a battery in the circuit. • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. Electrochemical cells have two conductive electrodes, called the anode and the cathode. In contrast, a galvanic cell has in its place either a. The anode is defined as the electrode where. We’ll discuss how these concepts are applied using different electrochemical cells. Within all electrochemical cells, there are 3 main components: • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit.
From www.shemmassianconsulting.com
Electrochemistry for the MCAT Everything You Need to Know Electrochemical Cell Mcat Below is a typical setup for an electrochemical cell! The anode is defined as the electrode where. In this article, we’ll go over everything you need to know about electrochemistry for the mcat. • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. • the main components of an electrolytic cell are. Electrochemical Cell Mcat.
From jackwestin.com
Electrolytic Cell Electrochemistry MCAT Content Electrochemical Cell Mcat Below is a typical setup for an electrochemical cell! In this article, we’ll go over everything you need to know about electrochemistry for the mcat. The anode is defined as the electrode where. On the diagram, this is represented by a battery in the circuit. • the key process of electrolysis is the interchange of atoms and ions by the. Electrochemical Cell Mcat.
From schoolbag.info
· 12.2 Electrochemical Cell Mcat We’ll discuss how these concepts are applied using different electrochemical cells. Within all electrochemical cells, there are 3 main components: • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. On the diagram, this is represented by a battery in the circuit. Electrochemical cells have two conductive electrodes, called the anode and. Electrochemical Cell Mcat.
From www.reddit.com
galvanic/electrolytic/concentration cells r/Mcat Electrochemical Cell Mcat In contrast, a galvanic cell has in its place either a. The anode is defined as the electrode where. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. • the key process of electrolysis is the. Electrochemical Cell Mcat.
From schoolbag.info
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell Electrochemical Cell Mcat In this article, we’ll go over everything you need to know about electrochemistry for the mcat. We’ll discuss how these concepts are applied using different electrochemical cells. Below is a typical setup for an electrochemical cell! Within all electrochemical cells, there are 3 main components: The anode is defined as the electrode where. Electrochemical cells have two conductive electrodes, called. Electrochemical Cell Mcat.
From www.reddit.com
electrochemical cells r/Mcat Electrochemical Cell Mcat • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. The anode is defined as the electrode where. Below is a typical setup for an electrochemical cell! Within all electrochemical cells, there are 3 main components: We’ll discuss how these concepts are applied using different electrochemical. Electrochemical Cell Mcat.
From www.youtube.com
MCAT Physics + Gen Chem How to Solve Electrochemical Cell MCAT Electrochemical Cell Mcat In this article, we’ll go over everything you need to know about electrochemistry for the mcat. On the diagram, this is represented by a battery in the circuit. • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. We’ll discuss how these concepts are applied using different electrochemical cells. Electrochemical cells have two conductive. Electrochemical Cell Mcat.
From jackwestin.com
Electrolytic Cell Electrochemistry MCAT Content Electrochemical Cell Mcat • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. Below is a typical setup for an electrochemical cell! • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. Electrochemical cells have two conductive electrodes, called the anode. Electrochemical Cell Mcat.
From schoolbag.info
Figure 12.2. Electrolysis of Molten NaCl Electrochemical Cell Mcat • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. The anode is defined as. Electrochemical Cell Mcat.
From www.reddit.com
Balancing electrochemical cells? r/Mcat Electrochemical Cell Mcat Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. In this article, we’ll go over everything you need. Electrochemical Cell Mcat.
From blog.blueprintprep.com
3 Concepts To Know About Electrochemical Cells for the MCAT MCAT Electrochemical Cell Mcat In this article, we’ll go over everything you need to know about electrochemistry for the mcat. We’ll discuss how these concepts are applied using different electrochemical cells. • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key. Electrochemical Cell Mcat.
From www.reddit.com
Electrochemical cells help!!! r/Mcat Electrochemical Cell Mcat The anode is defined as the electrode where. Below is a typical setup for an electrochemical cell! Electrochemical cells have two conductive electrodes, called the anode and the cathode. • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. • the main components of an electrolytic cell are an electrolyte, dc current,. Electrochemical Cell Mcat.
From www.reddit.com
Help with Electrochemical cells example question r/Mcat Electrochemical Cell Mcat Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. In contrast, a galvanic cell has in its place either a. The anode is defined as the electrode where. • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. Below is. Electrochemical Cell Mcat.
From www.reddit.com
How do I know if this is a galvanic or electrolytic cell r/Mcat Electrochemical Cell Mcat Below is a typical setup for an electrochemical cell! We’ll discuss how these concepts are applied using different electrochemical cells. • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. On the diagram, this is represented by a battery in the circuit. • the key process of electrolysis is the interchange of. Electrochemical Cell Mcat.
From www.reddit.com
galvanic/electrolytic/concentration cells r/Mcat Electrochemical Cell Mcat The anode is defined as the electrode where. • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. Within all electrochemical cells, there are 3 main components: Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. We’ll discuss how these concepts are. Electrochemical Cell Mcat.
From www.youtube.com
MCAT Question of the Day 16 Chemistry Electrochemical Cells YouTube Electrochemical Cell Mcat • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. Electrochemical cells have two conductive electrodes, called the anode and the cathode. • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. Within all electrochemical cells, there are 3 main. Electrochemical Cell Mcat.
From mcatmastery.net
Electrochemistry on the MCAT MCAT Mastery Electrochemical Cell Mcat We’ll discuss how these concepts are applied using different electrochemical cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Below is a typical setup for an electrochemical cell! Within all electrochemical cells, there are 3 main components: • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. In contrast, a. Electrochemical Cell Mcat.
From www.youtube.com
MCAT ElectrochemistryGalvanic cellsElectrolytic cellAnode, Cathode Electrochemical Cell Mcat In contrast, a galvanic cell has in its place either a. • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. We’ll discuss how these. Electrochemical Cell Mcat.
From www.reddit.com
AAMC Physics QPack 27 Electron Movement r/Mcat Electrochemical Cell Mcat In this article, we’ll go over everything you need to know about electrochemistry for the mcat. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. We’ll discuss how these concepts are applied using different electrochemical cells. • the key process of electrolysis is the interchange of atoms and. Electrochemical Cell Mcat.
From leah4sci.com
Electrochemistry Galvanic / Voltaic and Electrolytic Cells MCAT and Electrochemical Cell Mcat We’ll discuss how these concepts are applied using different electrochemical cells. • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. The anode is defined as the electrode where. Electrochemical cells have two conductive electrodes, called the anode and the cathode. • the main components of an electrolytic cell are an electrolyte,. Electrochemical Cell Mcat.
From webinar.cambridgecoaching.com
Electrochemical Cells The MCAT inar Electrochemical Cell Mcat Within all electrochemical cells, there are 3 main components: • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. On the diagram, this is represented by a battery in the circuit. Below is a typical setup for an electrochemical cell! Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic. Electrochemical Cell Mcat.
From www.reddit.com
Electrolytic cell electron movement r/Mcat Electrochemical Cell Mcat We’ll discuss how these concepts are applied using different electrochemical cells. In contrast, a galvanic cell has in its place either a. Electrochemical cells have two conductive electrodes, called the anode and the cathode. • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. In this article, we’ll go over everything you need to. Electrochemical Cell Mcat.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Electrochemical Cell Mcat Within all electrochemical cells, there are 3 main components: Below is a typical setup for an electrochemical cell! The anode is defined as the electrode where. • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. We’ll discuss how these concepts are applied using different electrochemical cells. In contrast, a galvanic cell has in. Electrochemical Cell Mcat.
From www.youtube.com
Electrochemistry Galvanic/Voltaic Cell Battery Made Super Simple! MCAT Electrochemical Cell Mcat • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. In this article, we’ll go over everything you need to know about electrochemistry for the mcat. We’ll discuss how these concepts are applied using different electrochemical cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. In contrast, a galvanic cell. Electrochemical Cell Mcat.
From www.reddit.com
In Electrochemical cells (Voltaic and Electrolytic) does the anode Electrochemical Cell Mcat Electrochemical cells have two conductive electrodes, called the anode and the cathode. The anode is defined as the electrode where. In contrast, a galvanic cell has in its place either a. On the diagram, this is represented by a battery in the circuit. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key. Electrochemical Cell Mcat.
From schoolbag.info
Figure 12.4. LeadAcid Battery When charged (a), the cell contains a Pb Electrochemical Cell Mcat The anode is defined as the electrode where. Below is a typical setup for an electrochemical cell! Within all electrochemical cells, there are 3 main components: • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. We’ll discuss how these concepts are applied using different electrochemical. Electrochemical Cell Mcat.
From www.youtube.com
MCAT Electrochemistry Voltaic Cell, Electrolytic Cell, Redox Reaction Electrochemical Cell Mcat • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. In this article, we’ll go over everything you need to know about electrochemistry for the mcat. The anode is defined as the electrode where. Electrochemical cells have two conductive electrodes, called the anode and the cathode.. Electrochemical Cell Mcat.
From www.reddit.com
Electrolytic cells r/Mcat Electrochemical Cell Mcat In this article, we’ll go over everything you need to know about electrochemistry for the mcat. • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. Within all electrochemical cells, there are 3 main components: Below is a typical setup for an electrochemical cell! • the key process of electrolysis is the interchange of. Electrochemical Cell Mcat.
From schoolbag.info
6. Fill in the following chart to summarize electrode charge Electrochemical Cell Mcat Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. The anode is defined as the electrode where. • the anode will undergo oxidation (loss of electrons) and reduction (gain of election) will happen at cathode. • the key process of electrolysis is the interchange of atoms and ions. Electrochemical Cell Mcat.
From www.youtube.com
MCAT Question of the Day Galvanic and Voltaic Cells YouTube Electrochemical Cell Mcat • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. We’ll discuss how these concepts are applied using different electrochemical cells. • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. Electrochemical cells have two conductive electrodes, called the anode. Electrochemical Cell Mcat.
From www.shemmassianconsulting.com
Electrochemistry for the MCAT Everything You Need to Know Electrochemical Cell Mcat • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. • the main components of an electrolytic cell are an electrolyte, dc current, and two. Electrochemical Cell Mcat.
From jackwestin.com
Concentration Cell Direction Of Electron Flow Nernst Equation Electrochemical Cell Mcat Within all electrochemical cells, there are 3 main components: In contrast, a galvanic cell has in its place either a. On the diagram, this is represented by a battery in the circuit. We’ll discuss how these concepts are applied using different electrochemical cells. Electrochemical cells have two conductive electrodes, called the anode and the cathode. Learn all about electrochemistry including. Electrochemical Cell Mcat.
From medlifemastery.com
Electrochemistry on the MCAT MedLife Mastery Electrochemical Cell Mcat • the main components of an electrolytic cell are an electrolyte, dc current, and two electrodes. In this article, we’ll go over everything you need to know about electrochemistry for the mcat. Below is a typical setup for an electrochemical cell! In contrast, a galvanic cell has in its place either a. We’ll discuss how these concepts are applied using. Electrochemical Cell Mcat.
From mygreexampreparation.com
Galvanic vs Electrolytic Cell MCAT (Electrochemistry Guide) Electrochemical Cell Mcat • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. In this article, we’ll go over everything you need to know about electrochemistry for the mcat. We’ll discuss how these concepts are applied using different electrochemical cells. In contrast, a galvanic cell has in its place. Electrochemical Cell Mcat.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Electrochemical Cell Mcat • the key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. On the diagram, this is represented by a battery in the circuit. Learn all about electrochemistry including the galvanic cell aka voltaic cell, and the electrolytic cell, a key topic in mcat chemistry. The anode is. Electrochemical Cell Mcat.