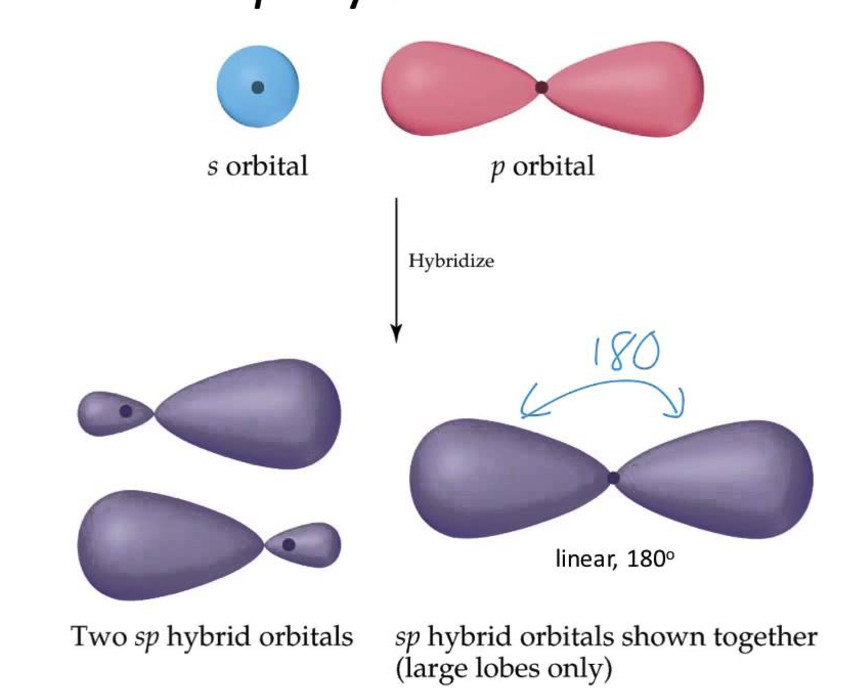
Hybridization Groups . Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. The geometry of the orbital.
from classnotes.org.in
Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. The geometry of the orbital. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral.
Hybridisation Chemical Bonding and Molecular Structure, Chemistry
Hybridization Groups Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The geometry of the orbital. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Hybridization Groups When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. The geometry of the orbital. Here’s a shortcut for how to determine the hybridization of an atom in. Hybridization Groups.
From www.slideserve.com
PPT Orbital Hybridization PowerPoint Presentation, free download ID Hybridization Groups Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. The five basic shapes. Hybridization Groups.
From www.masterorganicchemistry.com
What Are Hybrid Orbitals and Hybridization? Master Organic Chemistry Hybridization Groups Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. The geometry of the orbital. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Usually the hybridization on a certain atom can. Hybridization Groups.
From miguelgrorandolph.blogspot.com
What Is the Hybridization of the Se Atom in Sef6 Hybridization Groups Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. Hybridization of an s orbital with two p orbitals. Hybridization Groups.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Hybridization Groups The geometry of the orbital. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Here’s a shortcut for how to determine the. Hybridization Groups.
From avantecnica.qualitypoolsboulder.com
Hybridization Definition, Types, Rules, Examples Hybridization Groups Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each. Hybridization Groups.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Groups The geometry of the orbital. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three. Hybridization Groups.
From chemistrytalk.org
Hybridization and Hybrid Orbitals ChemTalk Hybridization Groups Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in. Hybridization Groups.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Hybridization Groups The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Usually the hybridization on a certain atom can simply be determined by counting the total. Hybridization Groups.
From www.slideserve.com
PPT Chemical Bonding and Nomenclature PowerPoint Presentation, free Hybridization Groups When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The geometry of the orbital. Here’s a shortcut for. Hybridization Groups.
From www.researchgate.net
Properties of hybridization groups as determined by different methods Hybridization Groups Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The geometry of the orbital. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Hybridization of an s orbital with two p orbitals (p x and p y) results in. Hybridization Groups.
From classnotes.org.in
Hybridisation Chemical Bonding and Molecular Structure, Chemistry Hybridization Groups The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. The geometry of the orbital. Hybridization of an s orbital with two p orbitals (p. Hybridization Groups.
From www.animalia-life.club
Hybridization Orbitals Chart Hybridization Groups Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The geometry of the orbital. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. Here’s a shortcut for. Hybridization Groups.
From www.geeksforgeeks.org
Hybridization Definition, Types, Rules, Examples Hybridization Groups The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The geometry of the orbital. When boron is in a molecule with three regions of electron density, three of the orbitals. Hybridization Groups.
From www.studypool.com
SOLUTION Overview of functional groups nomenclature of organic Hybridization Groups When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization of an. Hybridization Groups.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry Hybridization Groups The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. When boron is in a molecule with three regions of electron density, three. Hybridization Groups.
From www.youtube.com
How to determine Hybridization s, sp, sp2, and sp3 Organic Hybridization Groups The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. When boron is in a molecule with three regions of electron density, three. Hybridization Groups.
From www.windward.solutions
P4s3 hybridization Hybridization Groups Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. The geometry of the orbital. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three. Hybridization Groups.
From www.studypool.com
SOLUTION Overview of functional groups nomenclature of organic Hybridization Groups The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set. Hybridization Groups.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Groups The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. Usually the hybridization on a certain atom can simply be determined by counting. Hybridization Groups.
From techiescientist.com
SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram Hybridization Groups Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. Here’s. Hybridization Groups.
From www.youtube.com
Functional Groups and Hybridization Organic Chemistry 101 2 YouTube Hybridization Groups Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. The geometry of the orbital. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone. Hybridization Groups.
From techiescientist.com
XeCl4 Lewis Structure, Geometry, Hybridization, and Polarity Hybridization Groups Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set. Hybridization Groups.
From kpu.pressbooks.pub
1.6 Valence Bond Theory and Hybridization Organic Chemistry I Hybridization Groups Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. Usually the hybridization. Hybridization Groups.
From www.wizeprep.com
Hybridization Wize University Chemistry Textbook Wizeprep Hybridization Groups When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Usually the hybridization. Hybridization Groups.
From chemistryskills.com
Orbital Hybridization Chemistry Skills Hybridization Groups Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. Here’s a shortcut for. Hybridization Groups.
From www.youtube.com
Tips and Tricks for Easily Determining Hybridization States of Atoms in Hybridization Groups The geometry of the orbital. When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Usually the hybridization on a certain atom can simply be determined by counting. Hybridization Groups.
From www.pinterest.com
Hybridization Easy Science Chemistry education, Chemistry classroom Hybridization Groups The geometry of the orbital. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. Usually the hybridization on a certain atom can. Hybridization Groups.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry Hybridization Groups When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. The five basic. Hybridization Groups.
From in.pinterest.com
Orbital Hybridization "Cheat Sheet"? + Example Chemistry lessons Hybridization Groups The geometry of the orbital. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Hybridization of an s orbital with two p orbitals (p x and p y) results in. Hybridization Groups.
From www.organicchemistrytutor.com
Hybridization — Organic Chemistry Tutor Hybridization Groups The geometry of the orbital. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization of an s orbital with two p orbitals (p. Hybridization Groups.
From proper-cooking.info
Hybridization Periodic Table Hybridization Groups The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set. Hybridization Groups.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Hybridization Groups Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). The five basic shapes. Hybridization Groups.
From www.youtube.com
Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp Sp2 Sp3 YouTube Hybridization Groups Hybridization of an s orbital with two p orbitals (p x and p y) results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other (figure. The five basic shapes of hybridization are linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. When boron is in a molecule with three regions of electron density, three. Hybridization Groups.
From clarissa-bloggay.blogspot.com
How to Find Hybridization Around Central Atom Hybridization Groups When boron is in a molecule with three regions of electron density, three of the orbitals hybridize and create a set of three sp2 orbitals and one. The geometry of the orbital. Usually the hybridization on a certain atom can simply be determined by counting the total number of electron groups (bonding pairs and lone pairs). Hybridization of an s. Hybridization Groups.