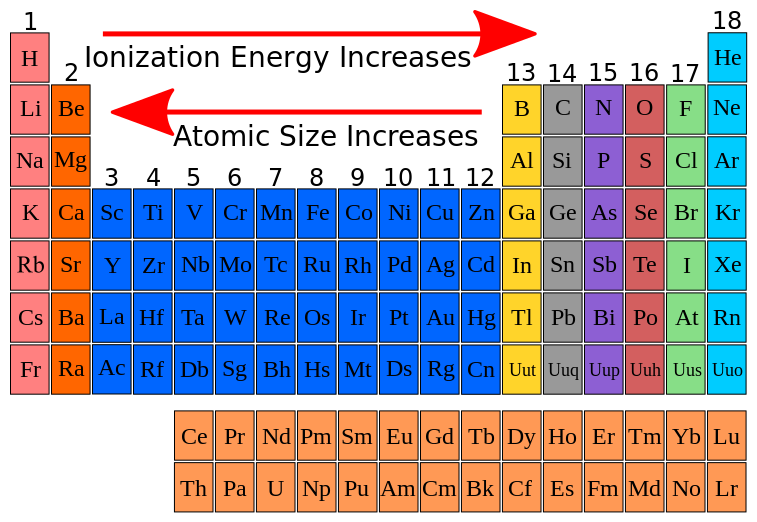
Metal Ionization Energy . First ionization energy, second ionization energy as well as. Ionization energy is the energy required to remove an electron from an atom or ion. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. Ionization energy increases moving across a period and decreases moving down a. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. 120 rows ionization energy chart of all the elements is given below. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. It is a minimum for the alkali metals which have a single electron. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. The first molar ionization energy applies to the.
from commons.wikimedia.org
Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. Ionization energy increases moving across a period and decreases moving down a. 120 rows ionization energy chart of all the elements is given below. The first molar ionization energy applies to the. Ionization energy is the energy required to remove an electron from an atom or ion. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. It is a minimum for the alkali metals which have a single electron. First ionization energy, second ionization energy as well as.
FileIonization energy atomic size.svg Wikimedia Commons
Metal Ionization Energy This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. The first molar ionization energy applies to the. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Ionization energy is the energy required to remove an electron from an atom or ion. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Ionization energy increases moving across a period and decreases moving down a. It is a minimum for the alkali metals which have a single electron.
From shaunmwilliams.com
Chapter 21 Presentation Metal Ionization Energy This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. 120 rows ionization energy chart of all the elements is given below. The first molar ionization energy applies to the. Ionization energy is. Metal Ionization Energy.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Metal Ionization Energy The first molar ionization energy applies to the. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. 120 rows ionization energy chart of all the elements is given below. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. This. Metal Ionization Energy.
From ar.inspiredpencil.com
Periodic Table Ionization Energy 3d Metal Ionization Energy Ionization energy increases moving across a period and decreases moving down a. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. The first molar ionization energy applies to the. Ionization energy is the energy required to remove an electron from a. Metal Ionization Energy.
From chem.libretexts.org
7.4 Ionization Energy Chemistry LibreTexts Metal Ionization Energy Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. The ionization energy is the quantity of energy that an isolated, gaseous atom in. Metal Ionization Energy.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend Metal Ionization Energy This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Ionization energy is the energy required to remove an electron from an atom or ion. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. The first molar ionization energy applies to the. 120 rows ionization. Metal Ionization Energy.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend Metal Ionization Energy The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. The first molar ionization energy applies to the. Ionization energy increases moving across a period and decreases moving down a. First ionization energy, second ionization energy as well as. The ionization energy or ionization potential is the energy necessary. Metal Ionization Energy.
From www.chemistrylearner.com
Ionization Energy Definition, Chart & Periodic Table Trend Metal Ionization Energy It is a minimum for the alkali metals which have a single electron. First ionization energy, second ionization energy as well as. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Ionization energy is the energy required to remove an electron. Metal Ionization Energy.
From chem.libretexts.org
8.4 Ionization Energy Chemistry LibreTexts Metal Ionization Energy The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the. Metal Ionization Energy.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki Metal Ionization Energy First ionization energy, second ionization energy as well as. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. It is a minimum for the alkali metals which have a single electron. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Ionization energy. Metal Ionization Energy.
From periodictableguide.com
Periodic table with Ionization Energy Values (Labeled Image) Metal Ionization Energy The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. Ionization energy increases moving across a period and decreases moving down a. It is a minimum for the alkali metals which have a single electron. Chemists define the ionization energy (\(i\)) of an element as the amount of energy. Metal Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Metal Ionization Energy This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. The first molar ionization energy applies to the. 120 rows ionization energy chart of all the elements is given below. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. It is a minimum for the. Metal Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Metal Ionization Energy First ionization energy, second ionization energy as well as. Ionization energy is the energy required to remove an electron from an atom or ion. 120 rows ionization energy chart of all the elements is given below. It is a minimum for the alkali metals which have a single electron. Ionization energy is the energy required to remove an electron from. Metal Ionization Energy.
From www.slideserve.com
PPT Chapter 24 Transition Metals and Coordination Compounds Metal Ionization Energy This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. The first molar ionization energy applies to the. Ionization energy increases moving across a period and decreases moving down a. 120 rows ionization energy chart of all the elements is given below. Ionization energy is the energy required to remove an electron from an. Metal Ionization Energy.
From www.slideserve.com
PPT Chapter 5 Periodic Law PowerPoint Presentation ID5165670 Metal Ionization Energy It is a minimum for the alkali metals which have a single electron. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. First ionization energy, second ionization energy as well as. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. The first molar ionization. Metal Ionization Energy.
From www.slideserve.com
PPT First Ionization Energies of Transition Metals PowerPoint Metal Ionization Energy First ionization energy, second ionization energy as well as. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. It is. Metal Ionization Energy.
From www.sliderbase.com
Periodic Trends Presentation Chemistry Metal Ionization Energy The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. 120 rows ionization energy chart of all the elements is given below. It is a minimum for the alkali metals which have a single electron. The first molar ionization energy applies to the. Ionization energy is the energy required to remove an. Metal Ionization Energy.
From alevelchemistry.co.uk
Ionisation Energy ALevel Chemistry Revision Notes Metal Ionization Energy This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Ionization energy increases moving across a period and decreases moving down a. 120 rows ionization energy chart of all the elements is given below. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. The ionization. Metal Ionization Energy.
From www.youtube.com
Metals First Ionisation Energy & Reactivity YouTube Metal Ionization Energy Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. This is the energy per mole necessary to remove electrons from gaseous atoms or. Metal Ionization Energy.
From tuitiontube.com
Ionization energy (or Ionisation energy) of group 1 (alkali metals Metal Ionization Energy Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Ionization energy is the energy required to remove an electron from an atom or ion. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral. Metal Ionization Energy.
From www.chemistrystudent.com
First Ionisation Energies (ALevel) ChemistryStudent Metal Ionization Energy Ionization energy increases moving across a period and decreases moving down a. Ionization energy is the energy required to remove an electron from an atom or ion. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral. Metal Ionization Energy.
From www.slideserve.com
PPT Transition Metals and Coordination Compounds PowerPoint Metal Ionization Energy It is a minimum for the alkali metals which have a single electron. 120 rows ionization energy chart of all the elements is given below. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. First ionization energy, second ionization energy as well as. Ionization energy is the energy. Metal Ionization Energy.
From therealgroupichem.weebly.com
Ionization energy Group i Chemistry(iiiescuro) Metal Ionization Energy Ionization energy increases moving across a period and decreases moving down a. The first molar ionization energy applies to the. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. First ionization energy, second. Metal Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Metal Ionization Energy Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. Chemists define the. Metal Ionization Energy.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Metal Ionization Energy Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. The first molar ionization energy applies to the. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground. Metal Ionization Energy.
From www.researchgate.net
Top The ionization energies for all ions of the first 12 metals Metal Ionization Energy First ionization energy, second ionization energy as well as. Ionization energy is the energy required to remove an electron from an atom or ion. The first molar ionization energy applies to the. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. This is the energy per mole necessary. Metal Ionization Energy.
From ideal.accelerate-ed.com
The Periodic Table and Ionization Energy Metal Ionization Energy Ionization energy is the energy required to remove an electron from an atom or ion. First ionization energy, second ionization energy as well as. Ionization energy increases moving across a period and decreases moving down a. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. Chemists define the. Metal Ionization Energy.
From commons.wikimedia.org
FileIonization energy atomic size.svg Wikimedia Commons Metal Ionization Energy It is a minimum for the alkali metals which have a single electron. First ionization energy, second ionization energy as well as. The first molar ionization energy applies to the. 120 rows ionization energy chart of all the elements is given below. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase.. Metal Ionization Energy.
From material-properties.org
Ionization Energy of Chemical Elements Material Properties Metal Ionization Energy Ionization energy increases moving across a period and decreases moving down a. Ionization energy is the energy required to remove an electron from an atom or ion. First ionization energy, second ionization energy as well as. 120 rows ionization energy chart of all the elements is given below. The first molar ionization energy applies to the. This is the energy. Metal Ionization Energy.
From byjus.com
Alkali Metals Properties, Electronic Configuration, Periodic Trends Metal Ionization Energy The first molar ionization energy applies to the. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. This is the energy per mole. Metal Ionization Energy.
From commons.wikimedia.org
FileIonization energy of alkali metals and alkaline earth metals.svg Metal Ionization Energy 120 rows ionization energy chart of all the elements is given below. Ionization energy is the energy required to remove an electron from an atom or ion. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic. Metal Ionization Energy.
From present5.com
University Chemistry Chapter 15 The Chemistry of Metal Ionization Energy This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. The first molar ionization energy applies to the. It is a minimum for the alkali metals which have a single electron. Ionization energy increases moving across a period and decreases moving down a. 120 rows ionization energy chart of all the elements is given. Metal Ionization Energy.
From ionizationpandai.blogspot.com
Ionization Metals Have Low Ionization Energy Metal Ionization Energy Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. First ionization energy, second ionization energy as well as. The first molar ionization energy applies to the. The ionization energy or ionization potential is the energy necessary to remove an electron from. Metal Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Metal Ionization Energy The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. Ionization energy is the energy required to remove an electron from an atom or ion. First ionization energy, second ionization energy as well as. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed. Metal Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Metal Ionization Energy It is a minimum for the alkali metals which have a single electron. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Ionization energy increases moving across a period and decreases moving down. Metal Ionization Energy.
From knordslearning.com
Ionization Energy Trend in Periodic Table (Explained) Metal Ionization Energy 120 rows ionization energy chart of all the elements is given below. Ionization energy is the energy required to remove an electron from an atom or ion. The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to. Ionization energy is the energy required to remove an electron from a. Metal Ionization Energy.