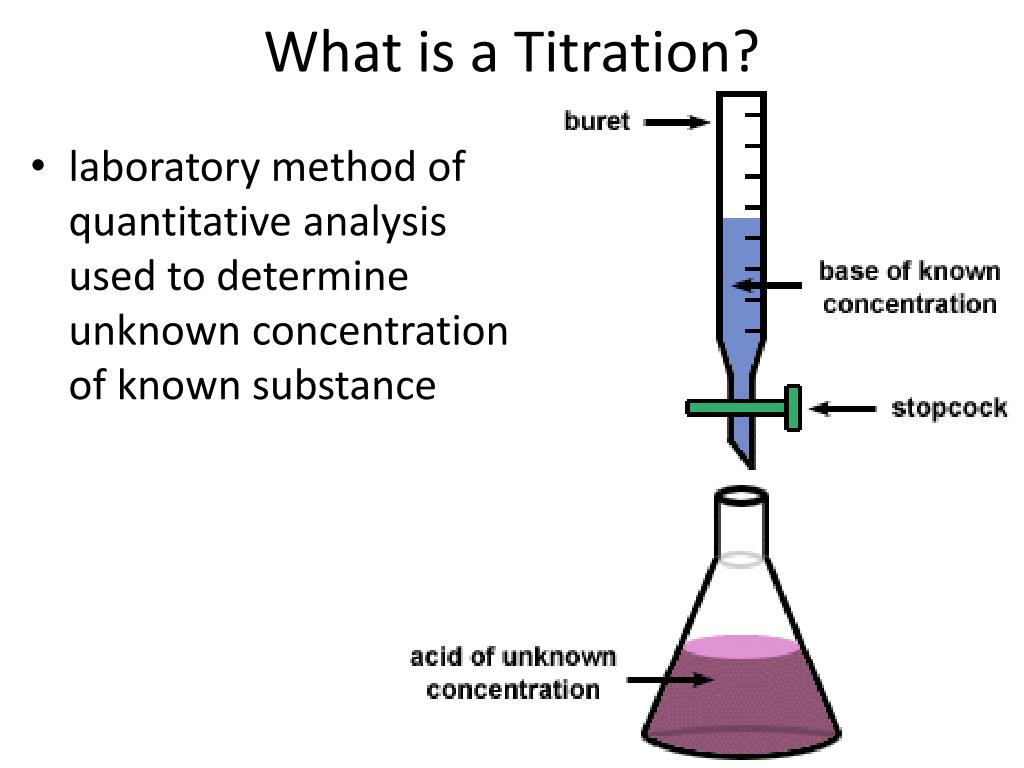
Titration Wash Bottle . Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. Delivers an accurate volume of solution into the conical flask for titration. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. A wash bottle of distilled water: This video explores all necessary information for preparing and. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. How to prepare for titration. Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. Read and record the position on the. We use these basic titrations to assess your. Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution.
from www.slideserve.com
Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. We use these basic titrations to assess your. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. How to prepare for titration. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution. Read and record the position on the. Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. This video explores all necessary information for preparing and. A wash bottle of distilled water:
PPT Neutralization Reactions using Titration Method PowerPoint
Titration Wash Bottle Delivers an accurate volume of solution into the conical flask for titration. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. This video explores all necessary information for preparing and. Delivers an accurate volume of solution into the conical flask for titration. How to prepare for titration. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. A wash bottle of distilled water: Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Read and record the position on the. We use these basic titrations to assess your. Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution.
From www.brand.de
Titration with the bottletop burette Titrette® Titration Wash Bottle Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. How to prepare for titration. Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with. Titration Wash Bottle.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Wash Bottle Delivers an accurate volume of solution into the conical flask for titration. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Using a direct titration procedure, you will prepare and standardize a solution of. Titration Wash Bottle.
From www.jadesci.com
Wash Bottle 250 mL "Water" White Titration Wash Bottle Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. Read and record the position on the. Delivers an accurate volume of solution into the conical flask for titration. Titration is a quantitative technique that uses a solution of. Titration Wash Bottle.
From www.shiners.com.au
Titration Kit Acidic Shiners Car Wash Systems Titration Wash Bottle A wash bottle of distilled water: How to prepare for titration. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution. 1.1 a titration involves measuring the exact volume of a reagent solution (the. Titration Wash Bottle.
From www.labfriend.com.au
Titration apparatus 251/10 without bottle LabFriend Australia Titration Wash Bottle Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. How to prepare for titration. Read and record the position on the. A wash bottle of distilled water: Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Delivers an accurate volume of solution into. Titration Wash Bottle.
From prowash.com.au
Self Serve and Automatic Car Wash Soap Titration Test Kit SuperSat Titration Wash Bottle This video explores all necessary information for preparing and. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. We use these basic titrations to assess your. 1.1 a. Titration Wash Bottle.
From scienceready.com.au
How to Make a Standard Solution HSC Chemistry Science Ready Titration Wash Bottle Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to. Titration Wash Bottle.
From prowash.com.au
Self Serve and Automatic Car Wash Soap Titration Test Kit SuperSat Titration Wash Bottle Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. This video explores all necessary information for preparing and. How to prepare for titration. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Use this to wash down the sides of the conical. Titration Wash Bottle.
From www.hannainst.com
Reagent Waste Bottle for HI903 and HI904 Karl Fischer Titration Systems Titration Wash Bottle Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. A wash bottle of distilled water: How to prepare for titration. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. Conduct practical investigations to analyse the concentration of an unknown acid or base by. Titration Wash Bottle.
From www.vitlab.com
Dropping bottles, PELD/PEHD VITLAB lab products (EN) Titration Wash Bottle Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. Delivers an accurate volume of solution into the conical. Titration Wash Bottle.
From www.youtube.com
Preparing for Titration Washing & Setup // HSC Chemistry YouTube Titration Wash Bottle Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Read and record the position on the. We. Titration Wash Bottle.
From www.studocu.com
Lab 7 Redox Titration Flowchart MATERIALS ☐ wash bottle, filled w Titration Wash Bottle Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; We use these basic titrations to assess your. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution. 1.1. Titration Wash Bottle.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Wash Bottle Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Read and record the position on the. This video explores all necessary information for preparing and. How to prepare for titration. We use these basic titrations to assess your. 1.1 a titration involves measuring the exact volume of a. Titration Wash Bottle.
From mavink.com
Titration Reaction Titration Wash Bottle A wash bottle of distilled water: Delivers an accurate volume of solution into the conical flask for titration. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Read and record the position on the. Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. Use this to wash. Titration Wash Bottle.
From edu.rsc.org
Mastering titration apparatus Poster RSC Education Titration Wash Bottle Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. This video explores all necessary information for preparing and. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that. Titration Wash Bottle.
From www.scharlab.com
Karl Fischer water content titration Scharlab Titration Wash Bottle Delivers an accurate volume of solution into the conical flask for titration. Read and record the position on the. Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution. Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. Using a direct titration. Titration Wash Bottle.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Wash Bottle Read and record the position on the. We use these basic titrations to assess your. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Conduct practical investigations to analyse the concentration of an. Titration Wash Bottle.
From www.dynalon.com
Burette Plastic Burette Dynalon Titration Wash Bottle This video explores all necessary information for preparing and. Read and record the position on the. Delivers an accurate volume of solution into the conical flask for titration. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it. Titration Wash Bottle.
From www.youtube.com
Titrette® Titration with the digital bottletop burette YouTube Titration Wash Bottle Read and record the position on the. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. A wash bottle of distilled water: Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. Delivers an accurate volume of solution into the conical flask. Titration Wash Bottle.
From scienceready.com.au
Titration Standard Solution, Washing, Setup HSC Chemistry Science Titration Wash Bottle We use these basic titrations to assess your. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. A wash bottle of distilled water: Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. This video explores all necessary information for preparing and. Read. Titration Wash Bottle.
From www.bio-circle.dk
Titration Kit small Titration Kit small BioCircle Denmark Titration Wash Bottle Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. A wash bottle of distilled water: Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution. Read and record the position on the. How to prepare for titration.. Titration Wash Bottle.
From www.electro-glo.com
Molybdenum Titrating Solution, OptiDrop Bottle ElectroGlo Distribution Titration Wash Bottle Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. This video explores all necessary information for preparing and. Titrate until the last drop of naoh solution. Titration Wash Bottle.
From carwashworld.com.au
Car Wash Chemistry Titration Testing Blendco Rhino Brite Plus Titration Wash Bottle Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. We use these basic titrations to assess your. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. How to prepare for titration. 1.1 a titration involves measuring the. Titration Wash Bottle.
From www.fishersci.com
Dynalon Automatic Glass Titrating BuretsBeakers, Bottles, Cylinders Titration Wash Bottle Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. A wash bottle of distilled water: 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Read and record the position on the. How to prepare for titration. Using a direct titration. Titration Wash Bottle.
From www.rudolphbros.com
Henkel Titrating Solution 61 Bottle 4L Titration Wash Bottle Delivers an accurate volume of solution into the conical flask for titration. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Using a direct titration procedure, you will prepare and standardize a solution. Titration Wash Bottle.
From www.brand.de
Titration with the bottletop burette Titrette® BRAND Titration Wash Bottle We use these basic titrations to assess your. A wash bottle of distilled water: Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Read and record the position on the. Titration is an. Titration Wash Bottle.
From www.prowash.com.au
Self Serve and Automatic Car Wash Soap Titration Test Kit SuperSat Titration Wash Bottle A wash bottle of distilled water: Titration is an analytical technique used to determine the concentration of an unknown solution by reacting it with a standard solution. Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. This video explores all necessary information for preparing and. Conduct practical investigations to analyse the concentration of. Titration Wash Bottle.
From www.fishersci.se
Buerkle™ Borosilicate Glass Automatic Titrating Burette with Storage Titration Wash Bottle Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. Read and record the position on the. This video explores all necessary information for preparing and. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Titration is a quantitative technique that uses a solution of known concentration to. Titration Wash Bottle.
From www.sliderbase.com
Titration Titration Wash Bottle Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Delivers an accurate volume. Titration Wash Bottle.
From www.electro-glo.com
PTS 2 Titrating Solution, OptiDrop Bottle ElectroGlo Distribution Titration Wash Bottle Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. This video explores all necessary information for preparing and. Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. Titrate until the last drop of naoh solution leaves a. Titration Wash Bottle.
From quizlet.com
lab and safety equipment Flashcards Quizlet Titration Wash Bottle Titrate until the last drop of naoh solution leaves a permanent pink colour in the solution. A wash bottle of distilled water: Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. How to prepare for titration. Using a direct titration procedure, you will prepare and standardize a solution of. Titration Wash Bottle.
From www.nagwa.com
Question Video Explaining Why the Conical Flask Is Placed on a White Titration Wash Bottle We use these basic titrations to assess your. Delivers an accurate volume of solution into the conical flask for titration. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Using a direct. Titration Wash Bottle.
From www.vitlab.com
Spray bottles, PP VITLAB lab products (EN) Titration Wash Bottle This video explores all necessary information for preparing and. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Use this to wash down the sides of the conical flask in case any reactant is stuck to the sides. We use these basic titrations to assess your. A wash bottle of distilled water: Using a. Titration Wash Bottle.
From www.thomassci.com
RighttoKnow, SafetyVented Wash Bottles with GHS Labeling Titration Wash Bottle We use these basic titrations to assess your. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Read and record the position on the. Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. This video explores all necessary information for preparing. Titration Wash Bottle.
From www.electro-glo.com
Sodium Molybdate Titrating Solution, OptiDrop Bottle ElectroGlo Titration Wash Bottle Using a direct titration procedure, you will prepare and standardize a solution of hydrochloric acid. Conduct practical investigations to analyse the concentration of an unknown acid or base by titration; Delivers an accurate volume of solution into the conical flask for titration. We use these basic titrations to assess your. 1.1 a titration involves measuring the exact volume of a. Titration Wash Bottle.