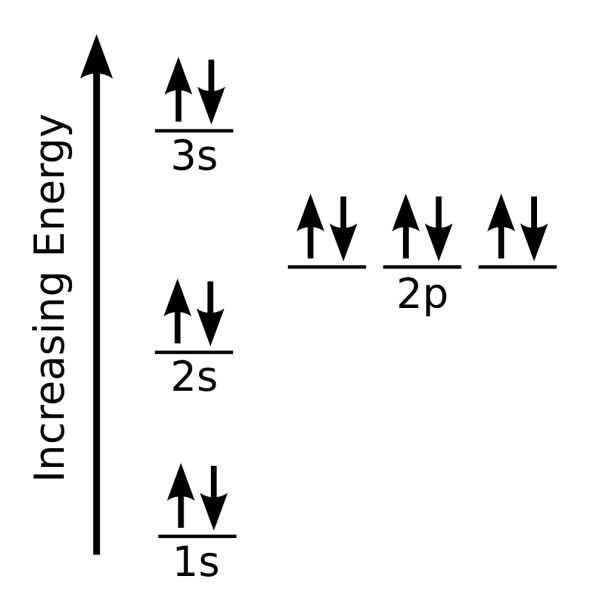
Magnesium Electron Configuration Abbreviated Form . The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration chart of all elements is mentioned in the table below. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2.
from www.mikrora.com
Electron configuration chart of all elements is mentioned in the table below. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. The shorthand electron configuration (or noble gas configuration) as well as.
File Electron Configuration Magnesium Svg Best Diagram Collection
Magnesium Electron Configuration Abbreviated Form In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. Magnesium has 12 electrons arranged in three energy levels. The shorthand electron configuration (or noble gas configuration) as well as. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons).
From brainly.in
Diagramatic sketch of electronic configuration of magnesium (atomic Magnesium Electron Configuration Abbreviated Form In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. Magnesium, a vital element for living organisms, has an atomic number of. Magnesium Electron Configuration Abbreviated Form.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Electron Configuration Abbreviated Form Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. The shorthand electron configuration (or noble gas configuration) as well as. Magnesium has 12 electrons arranged in three energy levels. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom. Magnesium Electron Configuration Abbreviated Form.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. In general, the electron configuration of an atom. Magnesium Electron Configuration Abbreviated Form.
From www.youtube.com
Magnesium electronic configuration How to Write Magnesium electronic Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of. Magnesium Electron Configuration Abbreviated Form.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Electron Configuration Abbreviated Form In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium has 12 electrons arranged in three energy levels. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium, a vital element for. Magnesium Electron Configuration Abbreviated Form.
From www.schoolmykids.com
Periodic Table Element Comparison Compare Magnesium vs Thorium Magnesium Electron Configuration Abbreviated Form The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In general, the electron. Magnesium Electron Configuration Abbreviated Form.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Electron Configuration Abbreviated Form Magnesium has 12 electrons arranged in three energy levels. Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there. Magnesium Electron Configuration Abbreviated Form.
From www.youtube.com
(a). Write down the electronic configuration of (i) magnesium atom, and Magnesium Electron Configuration Abbreviated Form Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. Magnesium has 12 electrons arranged in three energy levels. In order to. Magnesium Electron Configuration Abbreviated Form.
From www.coursehero.com
[Solved] Write the short form electron configuration for magnesium Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic. Magnesium Electron Configuration Abbreviated Form.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Electron Configuration Abbreviated Form The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium has 12 electrons arranged in three energy levels. The shorthand electron. Magnesium Electron Configuration Abbreviated Form.
From topblogtenz.com
Abbreviated electron configuration calculator [Noble gas] Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas. Magnesium Electron Configuration Abbreviated Form.
From www.youtube.com
Electron Configuration Sodium and Magnesium YouTube Magnesium Electron Configuration Abbreviated Form Electron configuration chart of all elements is mentioned in the table below. Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. The shorthand electron configuration (or noble gas configuration) as well as. In order to write the mg electron configuration we first need to know the number of electrons for. Magnesium Electron Configuration Abbreviated Form.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Configuration Abbreviated Form Electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Magnesium has 12 electrons arranged in three energy levels. In order to write the mg. Magnesium Electron Configuration Abbreviated Form.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Electron Configuration Abbreviated Form Magnesium has 12 electrons arranged in three energy levels. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in. Magnesium Electron Configuration Abbreviated Form.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Electron Configuration Abbreviated Form The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The shorthand electron configuration (or noble gas configuration) as well as. In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest. Magnesium Electron Configuration Abbreviated Form.
From www.numerade.com
SOLVED Write the complete electron configuration for the magnesium Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. Magnesium has 12 electrons arranged in three energy levels. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there. Magnesium Electron Configuration Abbreviated Form.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Magnesium Electron Configuration Abbreviated Form In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of mg2+ is. Magnesium Electron Configuration Abbreviated Form.
From www.numerade.com
SOLVED Write the abbreviated electron configurations for the elements Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The shorthand electron configuration (or noble gas configuration) as well as. In order to write the mg electron configuration we first need to know the number of. Magnesium Electron Configuration Abbreviated Form.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Magnesium Electron Configuration Abbreviated Form Electron configuration chart of all elements is mentioned in the table below. In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the. Magnesium Electron Configuration Abbreviated Form.
From periodiske-system.dk
Magnesium Magnesium Electron Configuration Abbreviated Form Electron configuration chart of all elements is mentioned in the table below. Magnesium has 12 electrons arranged in three energy levels. In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Magnesium Electron Configuration Abbreviated Form.
From www.youtube.com
Electronic configuration for Magnesium (Mg) spdf Trick Chemistry Magnesium Electron Configuration Abbreviated Form Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. In order to write the mg electron configuration we first need to. Magnesium Electron Configuration Abbreviated Form.
From herbana.world
Лекарственные растения и травы содержащие Магний. Описание, действие Magnesium Electron Configuration Abbreviated Form In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. The electron configuration of mg2+ is. Magnesium Electron Configuration Abbreviated Form.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Configuration Abbreviated Form Magnesium has 12 electrons arranged in three energy levels. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium, a vital. Magnesium Electron Configuration Abbreviated Form.
From montessorimuddle.org
atoms and molecules Page 2 Montessori Muddle Magnesium Electron Configuration Abbreviated Form Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In general, the electron configuration. Magnesium Electron Configuration Abbreviated Form.
From trueufiles204.weebly.com
Magnesium Valence Electrons trueufiles Magnesium Electron Configuration Abbreviated Form The shorthand electron configuration (or noble gas configuration) as well as. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. The electron configuration of magnesium. Magnesium Electron Configuration Abbreviated Form.
From www.pinterest.co.uk
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic. Magnesium Electron Configuration Abbreviated Form.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Magnesium Electron Configuration Abbreviated Form Magnesium has 12 electrons arranged in three energy levels. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration chart. Magnesium Electron Configuration Abbreviated Form.
From brainly.com
What is the correct electron configuration for magnesium? ( use Magnesium Electron Configuration Abbreviated Form In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration chart of all elements is mentioned in the table below. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne).. Magnesium Electron Configuration Abbreviated Form.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Magnesium Electron Configuration Abbreviated Form In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. The shorthand electron configuration (or noble gas configuration) as well as. In general, the electron configuration. Magnesium Electron Configuration Abbreviated Form.
From www.thoughtco.com
Magnesium Facts (Mg or Atomic Number 12) Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy. Magnesium Electron Configuration Abbreviated Form.
From valeriadoyle.blogspot.com
orbital diagram for magnesium ValeriaDoyle Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or noble gas configuration) as well as. In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning. Magnesium Electron Configuration Abbreviated Form.
From ar.inspiredpencil.com
Electron Configuration Magnesium Magnesium Electron Configuration Abbreviated Form In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Electron configuration chart of all elements is mentioned. Magnesium Electron Configuration Abbreviated Form.
From www.shutterstock.com
Diagram Representation Of The Element Magnesium Illustration Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium, a vital element for living organisms, has an atomic number of 12 and an electronic configuration of [ne]3s2. Magnesium has 12 electrons arranged in. Magnesium Electron Configuration Abbreviated Form.
From carisca.github.io
Write The Electronic Configuration Of Cr Orbital Diagram Electron 3d Magnesium Electron Configuration Abbreviated Form The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Magnesium has 12 electrons arranged in three energy levels. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble. Magnesium Electron Configuration Abbreviated Form.
From www.vrogue.co
Periodic Table Electrons Lost Or Gained Periodic Tabl vrogue.co Magnesium Electron Configuration Abbreviated Form The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron. Magnesium Electron Configuration Abbreviated Form.