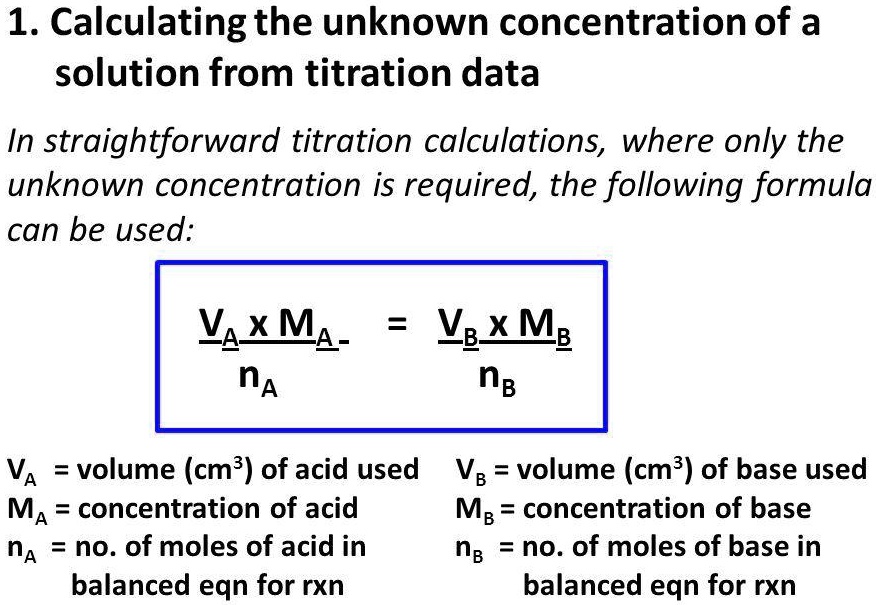
Titration Concentration Questions . Titration calculations & answers 1. A student uses a titration to determine the concentration of a sulfuric acid. In a titration, we measure the volume of an acid (or. Use the information to determine the concentration of the hydrochloric acid. Calculate the number of moles of barium hydroxide. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. Titrations are a very accurate way of measuring the concentration of acids and alkalis. The volumes of acids and alkali solutions that react with each other can be measured by. 13cm 3 of sodium hydroxide. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. It is a common laboratory technique that helps students understand chemical. The student starts by preparing a solution of sodium carbonate.
from www.vrogue.co
In a titration, we measure the volume of an acid (or. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. It is a common laboratory technique that helps students understand chemical. 13cm 3 of sodium hydroxide. A student uses a titration to determine the concentration of a sulfuric acid. The volumes of acids and alkali solutions that react with each other can be measured by. Calculate the number of moles of barium hydroxide. Use the information to determine the concentration of the hydrochloric acid. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Titration calculations & answers 1.
Titration Concentration Calculations Complete Lesson vrogue.co
Titration Concentration Questions It is a common laboratory technique that helps students understand chemical. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. Titration calculations & answers 1. Titrations are a very accurate way of measuring the concentration of acids and alkalis. In a titration, we measure the volume of an acid (or. A student uses a titration to determine the concentration of a sulfuric acid. The student starts by preparing a solution of sodium carbonate. The volumes of acids and alkali solutions that react with each other can be measured by. Calculate the number of moles of barium hydroxide. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. It is a common laboratory technique that helps students understand chemical. 13cm 3 of sodium hydroxide. Use the information to determine the concentration of the hydrochloric acid.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Concentration Questions The volumes of acids and alkali solutions that react with each other can be measured by. A student uses a titration to determine the concentration of a sulfuric acid. Titration calculations & answers 1. Calculate the number of moles of barium hydroxide. The student starts by preparing a solution of sodium carbonate. It is a common laboratory technique that helps. Titration Concentration Questions.
From www.numerade.com
SOLVED Question 84 The titration curve above represents the titration Titration Concentration Questions In a titration, we measure the volume of an acid (or. Calculate the number of moles of barium hydroxide. 13cm 3 of sodium hydroxide. The volumes of acids and alkali solutions that react with each other can be measured by. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. Titrations are a very accurate. Titration Concentration Questions.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Concentration Questions Titrations are a very accurate way of measuring the concentration of acids and alkalis. 13cm 3 of sodium hydroxide. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. Titration calculations & answers 1. In a titration, we measure the volume of an acid (or. Calculate the number of moles of barium hydroxide. Moles of. Titration Concentration Questions.
From www.youtube.com
Titration Calculations Past Exam Questions. YouTube Titration Concentration Questions Titration calculations & answers 1. Calculate the number of moles of barium hydroxide. Use the information to determine the concentration of the hydrochloric acid. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. The volumes of acids and alkali solutions that react with each other can be measured by. The student starts by preparing. Titration Concentration Questions.
From www.chegg.com
Solved Week 6 Lab Titration Concentration of Vinegar Titration Concentration Questions The volumes of acids and alkali solutions that react with each other can be measured by. It is a common laboratory technique that helps students understand chemical. A student uses a titration to determine the concentration of a sulfuric acid. Use the information to determine the concentration of the hydrochloric acid. 13cm 3 of sodium hydroxide. Titrations are a very. Titration Concentration Questions.
From classburns.z4.web.core.windows.net
Titration Practical Questions And Answers Titration Concentration Questions Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. In a titration, we measure the volume of an acid (or. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Titration calculations & answers 1. Use the information to determine the concentration of the hydrochloric acid. Moles of barium hydroxide. Titration Concentration Questions.
From www.youtube.com
TITRATION EXAM QUESTION for A LEVEL CHEMISTRY YouTube Titration Concentration Questions The student starts by preparing a solution of sodium carbonate. Use the information to determine the concentration of the hydrochloric acid. It is a common laboratory technique that helps students understand chemical. Titrations are a very accurate way of measuring the concentration of acids and alkalis. 13cm 3 of sodium hydroxide. A student uses a titration to determine the concentration. Titration Concentration Questions.
From louisvuittonsverige.cc
Concentration Worksheet Answer Key Solution Printable Worksheets and Titration Concentration Questions Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. It is a common laboratory technique that helps students understand chemical. The student starts by preparing a solution of sodium carbonate. Calculate the number of moles of barium hydroxide. Use the information to determine the concentration of the hydrochloric acid. The volumes of acids and. Titration Concentration Questions.
From www.youtube.com
A Simple Guide to Titration Calculations Part 1 Concentration YouTube Titration Concentration Questions Use the information to determine the concentration of the hydrochloric acid. A student uses a titration to determine the concentration of a sulfuric acid. In a titration, we measure the volume of an acid (or. Titrations are a very accurate way of measuring the concentration of acids and alkalis. 13cm 3 of sodium hydroxide. Titration calculations & answers 1. Titration. Titration Concentration Questions.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse Titration Concentration Questions The student starts by preparing a solution of sodium carbonate. In a titration, we measure the volume of an acid (or. A student uses a titration to determine the concentration of a sulfuric acid. Use the information to determine the concentration of the hydrochloric acid. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =.. Titration Concentration Questions.
From www.chegg.com
Solved Data Sheet Volumetric Analysis AcidBase Titration Titration Concentration Questions It is a common laboratory technique that helps students understand chemical. The student starts by preparing a solution of sodium carbonate. A student uses a titration to determine the concentration of a sulfuric acid. The volumes of acids and alkali solutions that react with each other can be measured by. Moles of barium hydroxide = concentration x volume (dm 3). Titration Concentration Questions.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Titration Concentration Questions 13cm 3 of sodium hydroxide. Use the information to determine the concentration of the hydrochloric acid. The student starts by preparing a solution of sodium carbonate. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. It is a common. Titration Concentration Questions.
From www.vrogue.co
Titration Concentration Calculations Complete Lesson vrogue.co Titration Concentration Questions Calculate the number of moles of barium hydroxide. In a titration, we measure the volume of an acid (or. A student uses a titration to determine the concentration of a sulfuric acid. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. 13cm 3 of sodium hydroxide. Titrations are a very accurate way of measuring. Titration Concentration Questions.
From www.bartleby.com
Answered In titration experiment, the titrant… bartleby Titration Concentration Questions It is a common laboratory technique that helps students understand chemical. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Titration calculations & answers 1. The volumes of acids and alkali solutions that react with each other can be measured. Titration Concentration Questions.
From www.chegg.com
Solved TITRATION. CONCENTRATION OF VINEGAR INTRODUCTION Titration Concentration Questions Use the information to determine the concentration of the hydrochloric acid. The volumes of acids and alkali solutions that react with each other can be measured by. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. 13cm 3 of sodium hydroxide. Titration is used in gcse chemistry to accurately determine the concentration of an. Titration Concentration Questions.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Titration Concentration Questions Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. It is a common laboratory technique that helps students understand chemical. In a titration, we measure the volume of an acid (or. The volumes of acids and alkali solutions that. Titration Concentration Questions.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Concentration Questions Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. 13cm 3 of sodium hydroxide. Titration calculations & answers 1. Use the information to determine the concentration of the hydrochloric acid. Calculate the number of moles of barium hydroxide. Titrations are a very accurate way of measuring the concentration of acids and alkalis. In a. Titration Concentration Questions.
From crunchchemistry.co.uk
Titration experimental technique questions Crunch Chemistry Titration Concentration Questions The volumes of acids and alkali solutions that react with each other can be measured by. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. Titrations are a very accurate way of measuring the concentration of acids and alkalis. A student uses a titration to determine the concentration of a sulfuric acid. It is. Titration Concentration Questions.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration Concentration Questions Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. It is a common laboratory technique that helps students understand chemical. The volumes of acids and alkali solutions that react with each other can be measured by. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. In a titration,. Titration Concentration Questions.
From www.learnsci.com
LearnSci Smart Worksheet Determine Concentration of HCl by Titration Titration Concentration Questions Titrations are a very accurate way of measuring the concentration of acids and alkalis. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. The student starts by preparing a solution of sodium carbonate. Calculate the number of moles of barium hydroxide. 13cm 3 of sodium hydroxide. Use the information to determine the concentration of. Titration Concentration Questions.
From www.numerade.com
SOLVED Question 8 1 pts The titration curve shown below represents a Titration Concentration Questions Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. 13cm 3 of sodium hydroxide. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Calculate the number of moles of barium hydroxide. Titration calculations & answers 1. Use the information to determine the concentration of the hydrochloric acid. The student. Titration Concentration Questions.
From www.chegg.com
Solved Use the following titration curve for the first four Titration Concentration Questions Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. A student uses a titration to determine the concentration of a sulfuric acid. Use the information to determine the concentration of the hydrochloric acid. Calculate the number of moles of barium hydroxide. It is a common laboratory technique that helps students understand chemical. The volumes. Titration Concentration Questions.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Concentration Questions Calculate the number of moles of barium hydroxide. Use the information to determine the concentration of the hydrochloric acid. 13cm 3 of sodium hydroxide. The volumes of acids and alkali solutions that react with each other can be measured by. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. Titrations are a very accurate. Titration Concentration Questions.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Concentration Questions Use the information to determine the concentration of the hydrochloric acid. Titration calculations & answers 1. A student uses a titration to determine the concentration of a sulfuric acid. 13cm 3 of sodium hydroxide. It is a common laboratory technique that helps students understand chemical. Calculate the number of moles of barium hydroxide. The student starts by preparing a solution. Titration Concentration Questions.
From www.tes.com
Titration concentration calculations lesson) Teaching Resources Titration Concentration Questions 13cm 3 of sodium hydroxide. Titration calculations & answers 1. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. Calculate the number of moles of barium hydroxide. The volumes of acids and alkali solutions that react with each other can be measured by. The student starts by preparing a solution of sodium carbonate. Use. Titration Concentration Questions.
From www.chegg.com
Solved TITRATION CONCENTRATION OF VINEGAR INTRODUCTION Titration Concentration Questions It is a common laboratory technique that helps students understand chemical. Use the information to determine the concentration of the hydrochloric acid. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. The student starts by preparing a solution of. Titration Concentration Questions.
From www.chegg.com
Solved QUESTION 1 A grade 12 learner performed a titration Titration Concentration Questions Titration calculations & answers 1. The student starts by preparing a solution of sodium carbonate. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. 13cm 3 of sodium hydroxide. The volumes of acids and alkali solutions that react with each other can be measured by. It is a common laboratory technique that helps students. Titration Concentration Questions.
From www.chegg.com
Solved CHEMISTRY. IDENTIFY WEAK ACID USING TITRATION CURVE A Titration Concentration Questions The volumes of acids and alkali solutions that react with each other can be measured by. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Moles of barium hydroxide = concentration x volume (dm 3) = 0.15 x 0.025 =. Use the information to determine the concentration of the hydrochloric acid. In a titration, we. Titration Concentration Questions.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Titration Concentration Questions Calculate the number of moles of barium hydroxide. 13cm 3 of sodium hydroxide. Titrations are a very accurate way of measuring the concentration of acids and alkalis. It is a common laboratory technique that helps students understand chemical. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. Use the information to determine the concentration. Titration Concentration Questions.
From www.chegg.com
Solved titration, the concentration of the titrant and the Titration Concentration Questions The volumes of acids and alkali solutions that react with each other can be measured by. Titration calculations & answers 1. 13cm 3 of sodium hydroxide. Calculate the number of moles of barium hydroxide. Use the information to determine the concentration of the hydrochloric acid. It is a common laboratory technique that helps students understand chemical. Titration is used in. Titration Concentration Questions.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Concentration Questions Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. 13cm 3 of sodium hydroxide. Use the information to determine the concentration of the hydrochloric acid. Titrations are a very accurate way of measuring the concentration of acids and alkalis. A student uses a titration to determine the concentration of a sulfuric acid. Moles of. Titration Concentration Questions.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Titration Concentration Questions Titrations are a very accurate way of measuring the concentration of acids and alkalis. The volumes of acids and alkali solutions that react with each other can be measured by. Calculate the number of moles of barium hydroxide. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. In a titration, we measure the volume. Titration Concentration Questions.
From www.numerade.com
[Tutorial Titration stoichiometry] This question will walk you through Titration Concentration Questions It is a common laboratory technique that helps students understand chemical. Titration calculations & answers 1. The volumes of acids and alkali solutions that react with each other can be measured by. Use the information to determine the concentration of the hydrochloric acid. Calculate the number of moles of barium hydroxide. Titration is used in gcse chemistry to accurately determine. Titration Concentration Questions.
From www.chegg.com
Solved TITRATION CONCENTRATION OF VINEGAR . INTRODUCTION Titration Concentration Questions Calculate the number of moles of barium hydroxide. Titration calculations & answers 1. Titrations are a very accurate way of measuring the concentration of acids and alkalis. In a titration, we measure the volume of an acid (or. Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. It is a common laboratory technique that. Titration Concentration Questions.
From www.numerade.com
SOLVED This method determines the chloride ion concentration of a Titration Concentration Questions Titration is used in gcse chemistry to accurately determine the concentration of an unknown solution. Titration calculations & answers 1. The volumes of acids and alkali solutions that react with each other can be measured by. A student uses a titration to determine the concentration of a sulfuric acid. In a titration, we measure the volume of an acid (or.. Titration Concentration Questions.