Purpose Of Evaporation In Chemistry . Evaporation causes the cooling exchange of energy during phase change. Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation is the process whereby atoms or molecules in a liquid state (or solid state if the substance sublimes) gain sufficient energy to. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Learn about evaporation in detail at byju's. The change from liquid phase to gas phase is called evaporation. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. If the water is instead kept in a closed. Learn its applications in an air cooler, an earthen pot. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Evaporation is the opposite of condensation.
from ar.inspiredpencil.com
The change from liquid phase to gas phase is called evaporation. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation is the opposite of condensation. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Learn about evaporation in detail at byju's. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation causes the cooling exchange of energy during phase change.
Evaporation Chemistry
Purpose Of Evaporation In Chemistry Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. Evaporation is the opposite of condensation. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. The change from liquid phase to gas phase is called evaporation. Learn about evaporation in detail at byju's. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Learn its applications in an air cooler, an earthen pot. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation causes the cooling exchange of energy during phase change. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Evaporation is the process whereby atoms or molecules in a liquid state (or solid state if the substance sublimes) gain sufficient energy to. If the water is instead kept in a closed. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase.
From techdiagrammer.com
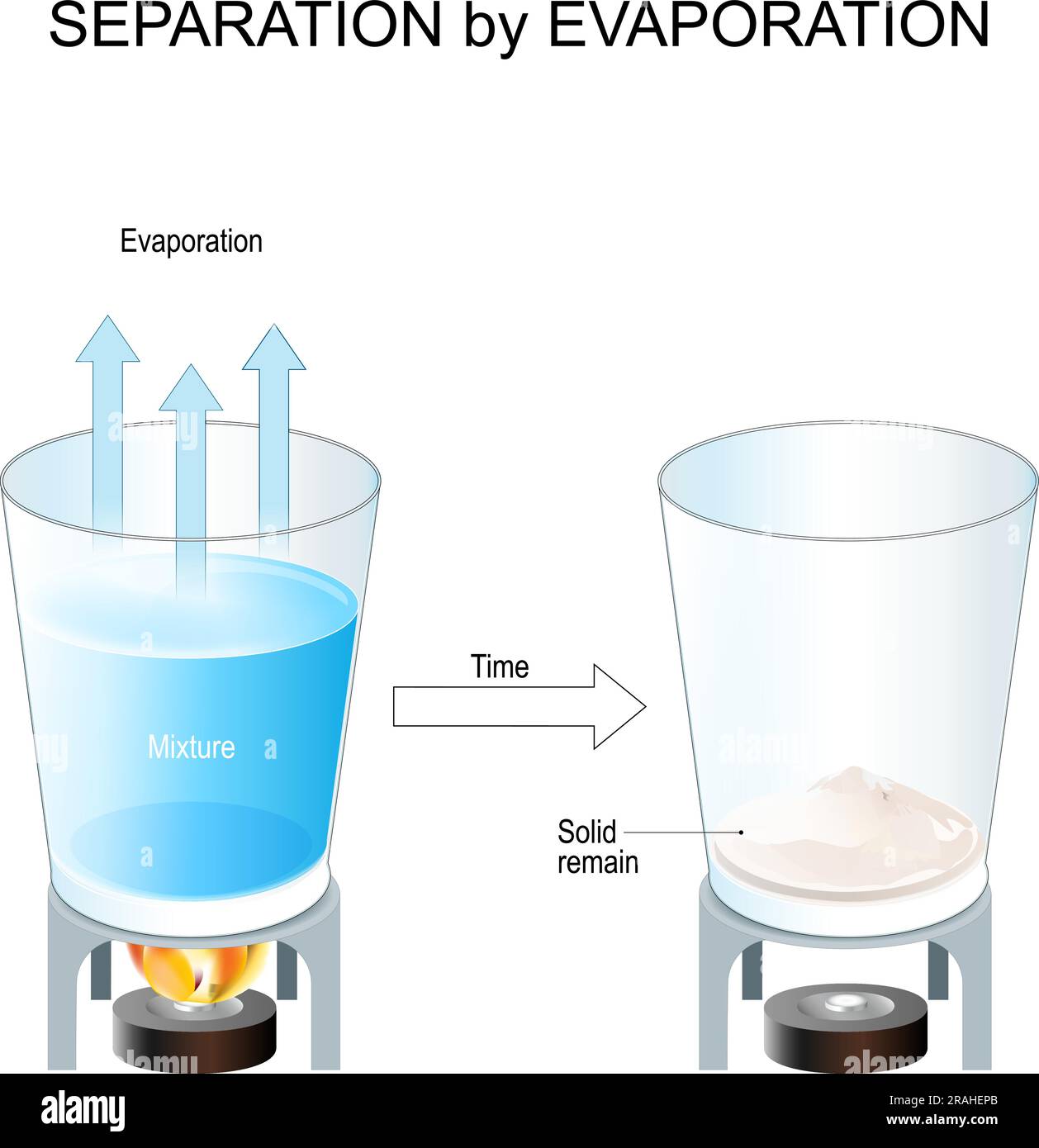
The Essential Guide to Understanding Evaporation Diagrams in Chemistry Purpose Of Evaporation In Chemistry Learn about evaporation in detail at byju's. Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. Evaporation is the opposite of condensation. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Learn its applications in. Purpose Of Evaporation In Chemistry.
From www.yaclass.in
Evaporation — lesson. Science CBSE, Class 9. Purpose Of Evaporation In Chemistry Learn about evaporation in detail at byju's. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. Evaporation is the process by which a liquid. Purpose Of Evaporation In Chemistry.
From www.tes.com
New KS3 Chemistry evaporation and condensation Teaching Resources Purpose Of Evaporation In Chemistry The change from liquid phase to gas phase is called evaporation. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Evaporation is the opposite of condensation. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly. Purpose Of Evaporation In Chemistry.
From www.youtube.com
Evaporation and Condensation Class 6th Chemistry YouTube Purpose Of Evaporation In Chemistry Evaporation is the opposite of condensation. Learn about evaporation in detail at byju's. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. If the water is instead kept in a closed. Evaporation, process. Purpose Of Evaporation In Chemistry.
From chemistnotes.com
Evaporation Definition, Process, and 5 Reliable Example Chemistry Notes Purpose Of Evaporation In Chemistry The change from liquid phase to gas phase is called evaporation. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Evaporation is the opposite. Purpose Of Evaporation In Chemistry.
From www.animalia-life.club
Evaporation Diagram Chemistry Purpose Of Evaporation In Chemistry Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Learn its applications in an air cooler, an earthen pot. The change from liquid phase to gas phase is called evaporation. Learn about. Purpose Of Evaporation In Chemistry.
From mavink.com
Labelled Diagram Of Evaporation Purpose Of Evaporation In Chemistry Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Learn about evaporation in detail at byju's. To isolate the solute, it would be a simple matter of. Purpose Of Evaporation In Chemistry.
From ar.inspiredpencil.com
Evaporation Chemistry Purpose Of Evaporation In Chemistry Evaporation is the opposite of condensation. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Evaporation, process by which an element or compound transitions. Purpose Of Evaporation In Chemistry.
From www.pinterest.com
Evaporation Evaporation, Basic, Techniques Purpose Of Evaporation In Chemistry Evaporation is the opposite of condensation. Learn its applications in an air cooler, an earthen pot. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. Evaporation is the process. Purpose Of Evaporation In Chemistry.
From www.teachoo.com
Why is Evaporation called a surface phenomenon? Teachoo Purpose Of Evaporation In Chemistry If the water is instead kept in a closed. Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. Evaporation is the process whereby atoms or molecules in a liquid state (or solid state if the substance sublimes) gain sufficient energy to. Learn about evaporation in detail at byju's. Evaporation is the process. Purpose Of Evaporation In Chemistry.
From ar.inspiredpencil.com
Evaporation Chemistry Purpose Of Evaporation In Chemistry For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Once the solvent had gone into the gas. Purpose Of Evaporation In Chemistry.
From mavink.com
Labelled Diagram Of Evaporation Purpose Of Evaporation In Chemistry Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. Evaporation is the opposite of condensation. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. To isolate the solute, it would be a simple matter of waiting. Purpose Of Evaporation In Chemistry.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Purpose Of Evaporation In Chemistry Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. Learn about evaporation in detail at byju's. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation is the opposite of condensation. If the water is instead kept in a closed. To isolate the solute,. Purpose Of Evaporation In Chemistry.
From techdiagrammer.com
The Essential Guide to Understanding Evaporation Diagrams in Chemistry Purpose Of Evaporation In Chemistry Evaporation is the process whereby atoms or molecules in a liquid state (or solid state if the substance sublimes) gain sufficient energy to. Evaporation is the opposite of condensation. Learn about evaporation in detail at byju's. The change from liquid phase to gas phase is called evaporation. Learn its applications in an air cooler, an earthen pot. Evaporation, process by. Purpose Of Evaporation In Chemistry.
From www.animalia-life.club
Evaporation Diagram Chemistry Purpose Of Evaporation In Chemistry It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. If the water is instead kept in a closed. Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. Evaporation is the process by which molecules undergo the. Purpose Of Evaporation In Chemistry.
From www.animalia-life.club
Evaporation Diagram Chemistry Purpose Of Evaporation In Chemistry Learn about evaporation in detail at byju's. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. The change from liquid phase to gas phase is called evaporation. Evaporation is the process by which a liquid transitions from the liquid phase to the gas. Purpose Of Evaporation In Chemistry.
From www.youtube.com
Class 9 Chemistry, Chapter 5, Lecture 19 Physical states of mater Purpose Of Evaporation In Chemistry If the water is instead kept in a closed. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. Evaporation is the process by which molecules undergo. Purpose Of Evaporation In Chemistry.
From www.animalia-life.club
Evaporation Diagram Chemistry Purpose Of Evaporation In Chemistry Evaporation is the opposite of condensation. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Learn its applications. Purpose Of Evaporation In Chemistry.
From www.tes.com
Evaporation and distillation KS3 Activate Science Teaching Resources Purpose Of Evaporation In Chemistry Evaporation is the opposite of condensation. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Learn its applications in an air cooler, an earthen pot. The change from. Purpose Of Evaporation In Chemistry.
From ar.inspiredpencil.com
Evaporation Chemistry Purpose Of Evaporation In Chemistry Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation is the opposite of condensation. If the water is instead kept in a closed. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Evaporation is the process whereby atoms or molecules. Purpose Of Evaporation In Chemistry.
From www.youtube.com
Separation of Substances Class 6 Science Chapter 5 Evaporation and Purpose Of Evaporation In Chemistry Evaporation is the opposite of condensation. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. If the water is instead kept in a closed. Evaporation causes the cooling exchange of energy during phase change. Once the solvent had gone into the gas phase. Purpose Of Evaporation In Chemistry.
From www.teachoo.com
How does Evaporation cause cooling? Explain (with Examples) Teachoo Purpose Of Evaporation In Chemistry Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Learn about evaporation in detail at byju's. If the water is instead kept in a closed. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. It is also how liquid water. Purpose Of Evaporation In Chemistry.
From www.toppr.com
Differences Between Evaporation and Boiling in Chemistry Purpose Of Evaporation In Chemistry Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. To isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Learn about evaporation in detail at byju's. Evaporation causes the cooling exchange of energy during phase change.. Purpose Of Evaporation In Chemistry.
From dustinajohnsono.blob.core.windows.net
Evaporation And Condensation Are Both Types Of Vaporization at Purpose Of Evaporation In Chemistry It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Evaporation is the opposite of condensation. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. If the water is instead kept in a. Purpose Of Evaporation In Chemistry.
From www.vrogue.co
Evaporation Illustration Twinkl vrogue.co Purpose Of Evaporation In Chemistry The change from liquid phase to gas phase is called evaporation. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Evaporation causes the cooling exchange of energy during phase change. If the water is instead kept in a closed. Evaporation is the process. Purpose Of Evaporation In Chemistry.
From www.bbc.co.uk
Evaporation BBC Bitesize Purpose Of Evaporation In Chemistry Evaporation causes the cooling exchange of energy during phase change. It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. For example, when humidity influences. Purpose Of Evaporation In Chemistry.
From www.slideserve.com
PPT Chemistry 30S PowerPoint Presentation, free download ID2819392 Purpose Of Evaporation In Chemistry For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. If the water is instead kept in a closed. Learn about evaporation in detail at byju's. Evaporation is the process whereby atoms or molecules in a liquid state (or solid state if the substance. Purpose Of Evaporation In Chemistry.
From www.studyiq.com
Evaporation and Condensation, Definition, Process, Types, Difference Purpose Of Evaporation In Chemistry For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Evaporation is the opposite of condensation. If the water is instead kept in a. Purpose Of Evaporation In Chemistry.
From techdiagrammer.com
The Essential Guide to Understanding Evaporation Diagrams in Chemistry Purpose Of Evaporation In Chemistry Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Learn about evaporation in detail at byju's. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Learn its applications in an air cooler, an earthen pot. Evaporation is. Purpose Of Evaporation In Chemistry.
From www.animalia-life.club
Evaporation Diagram Chemistry Purpose Of Evaporation In Chemistry Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Learn its applications in an air cooler, an earthen pot. Learn about evaporation in detail at byju's. Evaporation is the opposite of condensation. For example, when humidity influences the rate of evaporation of water, a high concentration of the. Purpose Of Evaporation In Chemistry.
From www.shutterstock.com
Chemistry Evaporation Diagram Vector Illutration Mixture Stock Vector Purpose Of Evaporation In Chemistry For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If the water is instead kept in a closed. Evaporation, process by which an element or compound. Purpose Of Evaporation In Chemistry.
From www.aplustopper.com
How can we Separate a Mixture of a Solid and a Liquid using Evaporation Purpose Of Evaporation In Chemistry For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. Evaporation is the opposite of condensation. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Learn its applications in an air cooler,. Purpose Of Evaporation In Chemistry.
From www.youtube.com
GCSE Chemistry Filtration, Evaporation & Crystallisation 6 YouTube Purpose Of Evaporation In Chemistry Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. If the water is instead kept in a closed. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation. To isolate the solute, it would. Purpose Of Evaporation In Chemistry.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Purpose Of Evaporation In Chemistry Evaporation causes the cooling exchange of energy during phase change. If the water is instead kept in a closed. Evaporation is the process whereby atoms or molecules in a liquid state (or solid state if the substance sublimes) gain sufficient energy to. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating. Purpose Of Evaporation In Chemistry.
From mavink.com
Evaporation Scientific Diagram Purpose Of Evaporation In Chemistry It is also how liquid water enters the atmosphere as water vapor, which is an important part of energy exchange that affects weather and climate. Evaporation is the process whereby atoms or molecules in a liquid state (or solid state if the substance sublimes) gain sufficient energy to. Evaporation is the process by which a liquid transitions from the liquid. Purpose Of Evaporation In Chemistry.