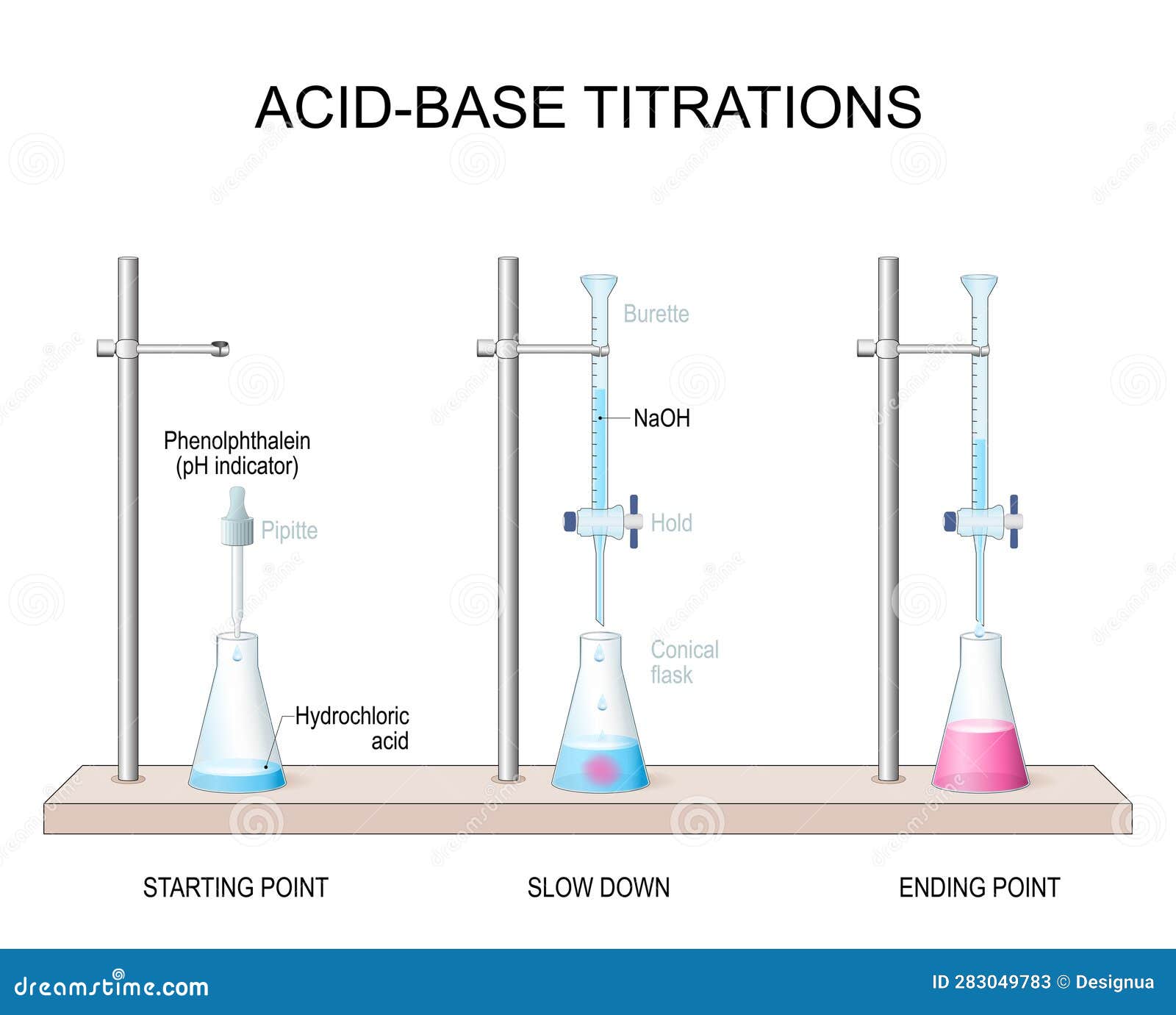
For Weak Acid Strong Base Titration The Indicator Used Is . Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. In this case, the weak acid is colourless and its ion is bright pink. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong. The molarity of the acid is given, so the number of moles titrated can be calculated: 0.050 l × 6 mol/l = 0.3 moles of strong acid. Compute sample ph at important stages of a titration. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Explain the function of acid.
from www.dreamstime.com
Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong. In this case, the weak acid is colourless and its ion is bright pink. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. 0.050 l × 6 mol/l = 0.3 moles of strong acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The molarity of the acid is given, so the number of moles titrated can be calculated: Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Compute sample ph at important stages of a titration. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Explain the function of acid.
Phenolphthalein Indicator in Acidbase Titration Stock Vector
For Weak Acid Strong Base Titration The Indicator Used Is Compute sample ph at important stages of a titration. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. In this case, the weak acid is colourless and its ion is bright pink. Explain the function of acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. 0.050 l × 6 mol/l = 0.3 moles of strong acid. Compute sample ph at important stages of a titration. The molarity of the acid is given, so the number of moles titrated can be calculated: Phenolphthalein is another commonly used indicator for titrations, and is another weak acid.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID For Weak Acid Strong Base Titration The Indicator Used Is Compute sample ph at important stages of a titration. Explain the function of acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid For Weak Acid Strong Base Titration The Indicator Used Is Compute sample ph at important stages of a titration. The molarity of the acid is given, so the number of moles titrated can be calculated: In this case, the weak acid is colourless and its ion is bright pink. 0.050 l × 6 mol/l = 0.3 moles of strong acid. Any of the three indicators will exhibit a reasonably sharp. For Weak Acid Strong Base Titration The Indicator Used Is.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 For Weak Acid Strong Base Titration The Indicator Used Is Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. In this case, the weak acid is colourless and its ion is bright pink. Explain. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive For Weak Acid Strong Base Titration The Indicator Used Is 0.050 l × 6 mol/l = 0.3 moles of strong acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong. In this case, the weak acid is colourless. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector For Weak Acid Strong Base Titration The Indicator Used Is Compute sample ph at important stages of a titration. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Explain the function of acid. The molarity of the acid is given, so the. For Weak Acid Strong Base Titration The Indicator Used Is.
From mungfali.com
Acid Base Titration Experiment For Weak Acid Strong Base Titration The Indicator Used Is Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. Explain the function of acid. The titration of a weak. For Weak Acid Strong Base Titration The Indicator Used Is.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration For Weak Acid Strong Base Titration The Indicator Used Is The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong. 0.050 l × 6 mol/l = 0.3 moles. For Weak Acid Strong Base Titration The Indicator Used Is.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts For Weak Acid Strong Base Titration The Indicator Used Is Explain the function of acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. The molarity of the acid is given, so. For Weak Acid Strong Base Titration The Indicator Used Is.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals For Weak Acid Strong Base Titration The Indicator Used Is The molarity of the acid is given, so the number of moles titrated can be calculated: The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or. For Weak Acid Strong Base Titration The Indicator Used Is.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration For Weak Acid Strong Base Titration The Indicator Used Is The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. 0.050 l × 6 mol/l = 0.3 moles of strong acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. In this case,. For Weak Acid Strong Base Titration The Indicator Used Is.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog For Weak Acid Strong Base Titration The Indicator Used Is The molarity of the acid is given, so the number of moles titrated can be calculated: Compute sample ph at important stages of a titration. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong. Ph indicators are weak acids or weak. For Weak Acid Strong Base Titration The Indicator Used Is.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors For Weak Acid Strong Base Titration The Indicator Used Is Compute sample ph at important stages of a titration. Explain the function of acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The molarity of the acid is given, so the. For Weak Acid Strong Base Titration The Indicator Used Is.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps For Weak Acid Strong Base Titration The Indicator Used Is Compute sample ph at important stages of a titration. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. Explain the function of acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. The. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.vecteezy.com
Acid base titration experiment and phases of color change during For Weak Acid Strong Base Titration The Indicator Used Is 0.050 l × 6 mol/l = 0.3 moles of strong acid. Compute sample ph at important stages of a titration. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators For Weak Acid Strong Base Titration The Indicator Used Is Compute sample ph at important stages of a titration. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Explain the function of acid. Any of the three indicators will exhibit a reasonably sharp color. For Weak Acid Strong Base Titration The Indicator Used Is.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve For Weak Acid Strong Base Titration The Indicator Used Is Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong. The molarity of the acid is given, so the number. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators For Weak Acid Strong Base Titration The Indicator Used Is Explain the function of acid. 0.050 l × 6 mol/l = 0.3 moles of strong acid. The molarity of the acid is given, so the number of moles titrated can be calculated: Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence. For Weak Acid Strong Base Titration The Indicator Used Is.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts For Weak Acid Strong Base Titration The Indicator Used Is Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. In this case, the weak acid is colourless and its ion is bright pink. Compute sample ph at important stages of a titration. The titration of a weak acid with a strong base involves the direct transfer. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations For Weak Acid Strong Base Titration The Indicator Used Is 0.050 l × 6 mol/l = 0.3 moles of strong acid. Explain the function of acid. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.slideshare.net
Acid base titration For Weak Acid Strong Base Titration The Indicator Used Is Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. 0.050 l × 6 mol/l = 0.3 moles of strong acid. Phenolphthalein is another commonly. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.sliderbase.com
Titration For Weak Acid Strong Base Titration The Indicator Used Is In this case, the weak acid is colourless and its ion is bright pink. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Compute sample ph at important stages of a titration. Ph indicators are weak acids or weak bases that have different colors based. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation For Weak Acid Strong Base Titration The Indicator Used Is In this case, the weak acid is colourless and its ion is bright pink. Compute sample ph at important stages of a titration. The molarity of the acid is given, so the number of moles titrated can be calculated: Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Any of the three indicators will exhibit a. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare For Weak Acid Strong Base Titration The Indicator Used Is Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. The molarity of the acid is given, so the number of moles titrated can be calculated: Compute sample ph at important stages of a titration. Any of the three indicators will exhibit a reasonably sharp color change at. For Weak Acid Strong Base Titration The Indicator Used Is.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple For Weak Acid Strong Base Titration The Indicator Used Is Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. Explain the function of acid. In this case, the weak acid is colourless and its ion is bright pink. Compute sample ph at important stages of a titration. 0.050 l × 6 mol/l = 0.3 moles of. For Weak Acid Strong Base Titration The Indicator Used Is.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators For Weak Acid Strong Base Titration The Indicator Used Is Explain the function of acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. 0.050 l × 6 mol/l = 0.3 moles of. For Weak Acid Strong Base Titration The Indicator Used Is.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps For Weak Acid Strong Base Titration The Indicator Used Is Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. 0.050 l × 6 mol/l = 0.3 moles of strong acid. Explain the function of acid. Compute sample ph at important stages of a titration.. For Weak Acid Strong Base Titration The Indicator Used Is.
From mmerevise.co.uk
pH Curves Questions and Revision MME For Weak Acid Strong Base Titration The Indicator Used Is Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. The molarity of the acid is given, so the number of moles titrated can be calculated: Compute sample ph at important stages of a titration. Determine ph and concentrations of chemical species at any point in the titration. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.narodnatribuna.info
Sparknotes Titrations Acidbase Titrations For Weak Acid Strong Base Titration The Indicator Used Is The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Compute sample ph at important stages of a titration. Any of the three indicators. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.hotzxgirl.com
Solved Choose The Correct Indicator For Acid Base Titration Chegg Hot For Weak Acid Strong Base Titration The Indicator Used Is The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. 0.050 l × 6 mol/l = 0.3 moles of strong acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The molarity of the acid is given, so the number of moles. For Weak Acid Strong Base Titration The Indicator Used Is.
From mungfali.com
Acid Base Titration Indicator For Weak Acid Strong Base Titration The Indicator Used Is Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Determine ph and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong. 0.050 l × 6 mol/l = 0.3 moles of strong. For Weak Acid Strong Base Titration The Indicator Used Is.
From byjus.com
Why is phenopthalein not used as an indicator in the titration of weak For Weak Acid Strong Base Titration The Indicator Used Is Compute sample ph at important stages of a titration. The molarity of the acid is given, so the number of moles titrated can be calculated: In this case, the weak acid is colourless and its ion is bright pink. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration,. For Weak Acid Strong Base Titration The Indicator Used Is.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept For Weak Acid Strong Base Titration The Indicator Used Is Compute sample ph at important stages of a titration. 0.050 l × 6 mol/l = 0.3 moles of strong acid. In this case, the weak acid is colourless and its ion is bright pink. The molarity of the acid is given, so the number of moles titrated can be calculated: Explain the function of acid. Any of the three indicators. For Weak Acid Strong Base Titration The Indicator Used Is.
From mungfali.com
Acid Base Titration Procedure For Weak Acid Strong Base Titration The Indicator Used Is The molarity of the acid is given, so the number of moles titrated can be calculated: Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Compute sample ph at important stages of. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID2134619 For Weak Acid Strong Base Titration The Indicator Used Is 0.050 l × 6 mol/l = 0.3 moles of strong acid. Ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic environments. Any of the three indicators will exhibit a reasonably sharp color change at the equivalence point of the strong acid titration, but only. In this case, the. For Weak Acid Strong Base Titration The Indicator Used Is.
From www.cloudizsexy.com
Acid Base Titration Titration Curves Equivalence Point And Indicators For Weak Acid Strong Base Titration The Indicator Used Is The molarity of the acid is given, so the number of moles titrated can be calculated: The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. 0.050 l × 6 mol/l = 0.3 moles of strong acid. Any of the three indicators will exhibit a reasonably. For Weak Acid Strong Base Titration The Indicator Used Is.