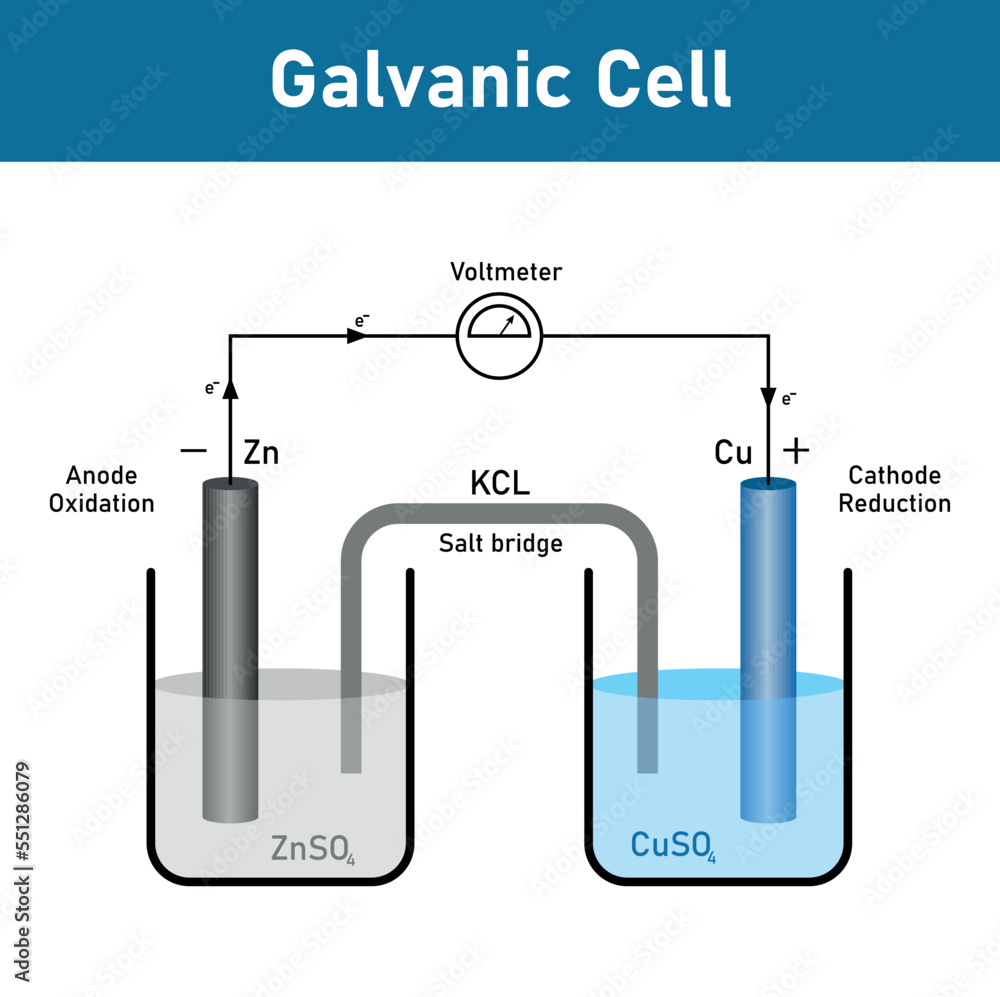
Zinc And Copper Electrochemical Cell . A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. In the process of the reaction,. A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. The balanced chemical equation is as follows: A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+} (aq) + cu(s)} \label{20.3.4} \] To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons.
from stock.adobe.com
A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+} (aq) + cu(s)} \label{20.3.4} \] The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The balanced chemical equation is as follows: As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. In the process of the reaction,.
Electrochemical cell diagram. Galvanic cell or voltaic cell. Zinc anode
Zinc And Copper Electrochemical Cell In the process of the reaction,. In the process of the reaction,. The balanced chemical equation is as follows: A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+} (aq) + cu(s)} \label{20.3.4} \] As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Zinc And Copper Electrochemical Cell A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. To illustrate the basic principles of a galvanic cell, let’s consider the. Zinc And Copper Electrochemical Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Zinc And Copper Electrochemical Cell In the process of the reaction,. As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. The balanced chemical equation is as follows: A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. A simple electrochemical cell can be made from. Zinc And Copper Electrochemical Cell.
From www.youtube.com
ZincCopper Electrochemical Cell Demonstration YouTube Zinc And Copper Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. The two solutions are separated by a porous. Zinc And Copper Electrochemical Cell.
From www.slideserve.com
PPT Chapter 22 Electrochemistry PowerPoint Presentation, free Zinc And Copper Electrochemical Cell \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+} (aq) + cu(s)} \label{20.3.4} \] A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. In the process of the reaction,. A basic voltaic cell is created by immersing in a diluted solution. Zinc And Copper Electrochemical Cell.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Zinc And Copper Electrochemical Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. The balanced chemical equation is as follows: To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic. Zinc And Copper Electrochemical Cell.
From stock.adobe.com
Illustration of galvanic cell consists of zinc and copper. Stock Vector Zinc And Copper Electrochemical Cell The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. A simple electrochemical cell can be made from copper and zinc metals with solutions of. Zinc And Copper Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Zinc And Copper Electrochemical Cell A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. A typical cell might consist of. Zinc And Copper Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5405206 Zinc And Copper Electrochemical Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+} (aq) + cu(s)} \label{20.3.4} \] As the above video shows, placing. Zinc And Copper Electrochemical Cell.
From www.vrogue.co
Solved 1 The Diagram Shows An Electrochemical Cell Wi vrogue.co Zinc And Copper Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. One reactant gives up electrons (undergoes oxidation) and. Zinc And Copper Electrochemical Cell.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Zinc And Copper Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The balanced chemical equation is as follows: A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow. Zinc And Copper Electrochemical Cell.
From saylordotorg.github.io
Electrochemistry Zinc And Copper Electrochemical Cell A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. In the process of the reaction,. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with. Zinc And Copper Electrochemical Cell.
From www.scienceabc.com
(Photo Credit Nandalal Sarkar/Shutterstock) Zinc And Copper Electrochemical Cell The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. In the process of the reaction,. One reactant gives up electrons (undergoes oxidation) and another reactant gains. Zinc And Copper Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Zinc And Copper Electrochemical Cell The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. The balanced chemical equation is as follows: One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. In the process of the reaction,. A simple electrochemical cell can be made from copper and zinc metals with solutions. Zinc And Copper Electrochemical Cell.
From www.slideserve.com
PPT What is an Electrochemical Cell? PowerPoint Presentation, free Zinc And Copper Electrochemical Cell The balanced chemical equation is as follows: A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. In the process of the. Zinc And Copper Electrochemical Cell.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Zinc And Copper Electrochemical Cell A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through.. Zinc And Copper Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Zinc And Copper Electrochemical Cell A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A simple electrochemical cell can be made from copper and zinc metals with solutions of. Zinc And Copper Electrochemical Cell.
From tecnico.aspillagahornauer.cl
Electrochemical Cells, 41 OFF Zinc And Copper Electrochemical Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. In the process of the reaction,. The balanced chemical equation is as follows: A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A simple electrochemical cell can. Zinc And Copper Electrochemical Cell.
From rohanfersmorrison.blogspot.com
Identify the Conditions for a Standard Electrochemical Cell. Zinc And Copper Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. In the process of the reaction,. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+} (aq) + cu(s)} \label{20.3.4} \] The balanced chemical equation is as follows: To illustrate the basic principles. Zinc And Copper Electrochemical Cell.
From www.vrogue.co
Simple Electrochemical Or Galvanic Cell The Daniell C vrogue.co Zinc And Copper Electrochemical Cell The balanced chemical equation is as follows: A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+} (aq) + cu(s)} \label{20.3.4} \] A voltaic cell is an electrochemical cell that produces electrical energy. Zinc And Copper Electrochemical Cell.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Zinc And Copper Electrochemical Cell As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. A typical cell might consist of two pieces of metal, one zinc and the. Zinc And Copper Electrochemical Cell.
From www.alamy.com
Copper zinc cell Cut Out Stock Images & Pictures Alamy Zinc And Copper Electrochemical Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. In the process of the reaction,. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a. Zinc And Copper Electrochemical Cell.
From www.shutterstock.com
Zinc Copper Electrochemical Cell Stock Vector (Royalty Free) 1340295668 Zinc And Copper Electrochemical Cell As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. A typical cell might consist of two pieces of metal, one zinc and the other. Zinc And Copper Electrochemical Cell.
From chem.libretexts.org
19.5 Standard Electrochemical Potentials Chemistry LibreTexts Zinc And Copper Electrochemical Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. In the process of the reaction,. A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +). Zinc And Copper Electrochemical Cell.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Zinc And Copper Electrochemical Cell To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. A voltaic cell is an. Zinc And Copper Electrochemical Cell.
From www.freepik.com
Premium Photo Electrochemical cell or Galvanic cell. The Daniell cell Zinc And Copper Electrochemical Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The balanced chemical equation is as follows: To illustrate the basic principles of a galvanic cell, let’s consider. Zinc And Copper Electrochemical Cell.
From stock.adobe.com
Electrochemical cell diagram. Galvanic cell or voltaic cell. Zinc anode Zinc And Copper Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 +. Zinc And Copper Electrochemical Cell.
From www.vrogue.co
Solved 1 The Diagram Shows An Electrochemical Cell Wi vrogue.co Zinc And Copper Electrochemical Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The balanced chemical equation is as follows: A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. One reactant gives up electrons (undergoes. Zinc And Copper Electrochemical Cell.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Electrochemical Cell In the process of the reaction,. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The balanced chemical equation is as follows: The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows. Zinc And Copper Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID1195562 Zinc And Copper Electrochemical Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A voltaic cell is an electrochemical cell that produces electrical energy using a chemical reaction. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. \[\ce{zn (s) +. Zinc And Copper Electrochemical Cell.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Zinc And Copper Electrochemical Cell To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+} (aq) + cu(s)} \label{20.3.4} \] A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc. Zinc And Copper Electrochemical Cell.
From ar.inspiredpencil.com
Copper Electrolytic Cell Zinc And Copper Electrochemical Cell A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. In the process of the reaction,. The two. Zinc And Copper Electrochemical Cell.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Zinc And Copper Electrochemical Cell As the above video shows, placing zinc metal into the copper(ii) solution results in a spontaneous redox reaction (\(\delta g <0\)), and if we. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. In the process of the reaction,.. Zinc And Copper Electrochemical Cell.
From gaskatel.de
Principles of electrochemical cells Gaskatel Zinc And Copper Electrochemical Cell A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (cu 2 +) to give copper metal and zn 2 + ion. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+}. Zinc And Copper Electrochemical Cell.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Zinc And Copper Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. A basic voltaic cell is created by immersing in a diluted solution of. Zinc And Copper Electrochemical Cell.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER Zinc And Copper Electrochemical Cell A basic voltaic cell is created by immersing in a diluted solution of sulfuric acid a zinc plate and a copper plate. The two solutions are separated by a porous barrier that prevents them from rapidly mixing but allows ions to diffuse through. \[\ce{zn (s) + cu^{2+} (aq) \rightarrow zn^{2+} (aq) + cu(s)} \label{20.3.4} \] To illustrate the basic principles. Zinc And Copper Electrochemical Cell.