Electrodes Used In Car Batteries . One way to understand electrochemical cells is to consider them in two commonly used items: The positive anode tends to be made up of. This energy leaves the battery. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. At the most basic level,. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices.
from batteryworld.varta-automotive.com
One way to understand electrochemical cells is to consider them in two commonly used items: At the most basic level,. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. The positive anode tends to be made up of. This energy leaves the battery. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function.
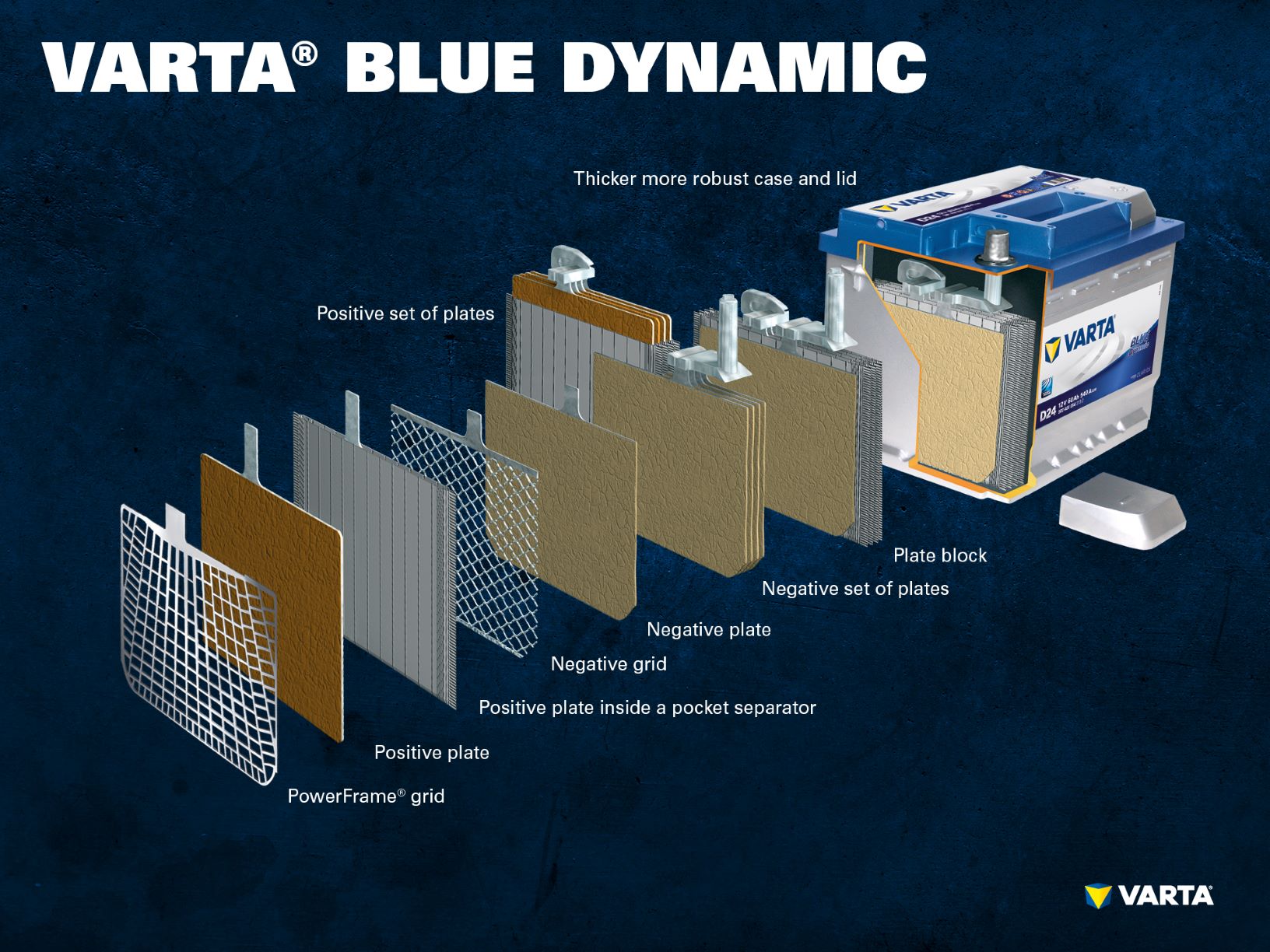
How does a car battery work and how is it constructed?
Electrodes Used In Car Batteries In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. At the most basic level,. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. One way to understand electrochemical cells is to consider them in two commonly used items: This energy leaves the battery. The positive anode tends to be made up of. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices.
From www.batterypowertips.com
What is an electrolyte? Battery Power Tips Electrodes Used In Car Batteries In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. This energy leaves the battery. At. Electrodes Used In Car Batteries.
From www2.mdpi.com
Batteries Free FullText Critical Review of the Use of Reference Electrodes in LiIon Electrodes Used In Car Batteries In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. The positive anode tends to be made up of. This energy leaves the battery. One way to understand electrochemical cells is to consider them in two commonly used items: Electric car batteries rely on the interplay of several. Electrodes Used In Car Batteries.
From www.mach1services.com
How does a car battery work? Mach 1 Services Electrodes Used In Car Batteries At the most basic level,. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. The positive anode tends to be made up of. This energy leaves the battery. One way to understand electrochemical cells is to consider them in two commonly used items: In general, a battery cell is made. Electrodes Used In Car Batteries.
From www.biologic.net
Anode vs Cathode What's the difference? BioLogic Electrodes Used In Car Batteries The positive anode tends to be made up of. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering. Electrodes Used In Car Batteries.
From arstechnica.com
New electrode material could lead to rechargeable sodium batteries Ars Technica Electrodes Used In Car Batteries In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. One way to understand electrochemical cells is to consider them in two commonly used items: This energy leaves the battery. At the most basic level,.. Electrodes Used In Car Batteries.
From www.autocar.co.uk
Carbon electrodes promise EV battery performance breakthrough Autocar Electrodes Used In Car Batteries The positive anode tends to be made up of. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. At the most basic level,. One way to understand electrochemical. Electrodes Used In Car Batteries.
From www.hioki.com
What are the Electrode Sheets that Greatly Affect the Quality of Lithiumion Batteries? Electrodes Used In Car Batteries This energy leaves the battery. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. The positive anode tends to be made up of. At the most basic level,. In general, a battery cell is. Electrodes Used In Car Batteries.
From us.misumi-ec.com
How Electric Vehicle Battery Packs are Manufactured MISUMI Mech Lab Blog Electrodes Used In Car Batteries At the most basic level,. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. The positive anode tends to be made up of. This energy leaves the battery. In general, a battery cell is. Electrodes Used In Car Batteries.
From www.azonano.com
UltraFast Carbon Electrodes for EV Batteries Electrodes Used In Car Batteries One way to understand electrochemical cells is to consider them in two commonly used items: Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. At the most basic level,. The positive anode tends to be made up of. In general, a battery cell is made up of an anode, cathode,. Electrodes Used In Car Batteries.
From www.alamy.com
Positive electrode Stock Vector Images Alamy Electrodes Used In Car Batteries The positive anode tends to be made up of. At the most basic level,. This energy leaves the battery. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. One way to understand electrochemical cells. Electrodes Used In Car Batteries.
From thenextweb.com
Researchers use water to create a safer and more durable EV battery cell Electrodes Used In Car Batteries The positive anode tends to be made up of. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which. Electrodes Used In Car Batteries.
From www.autocar.co.uk
Carbon electrodes promise EV battery performance breakthrough Autocar Electrodes Used In Car Batteries At the most basic level,. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. One way to understand electrochemical cells is to consider them in two commonly used items: In general, a battery cell. Electrodes Used In Car Batteries.
From fixcarbattery.blogspot.com
Recondition Car battery Magnesium copper water battery Electrodes Used In Car Batteries In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. At the most basic level,. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. The positive anode tends to be made up of. In a study published june. Electrodes Used In Car Batteries.
From batteryworld.varta-automotive.com
How does a car battery work and how is it constructed? Electrodes Used In Car Batteries At the most basic level,. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. This energy leaves the battery. The positive anode tends to be made up of. One way to understand electrochemical cells is to consider them in two commonly used items: Electric car batteries rely. Electrodes Used In Car Batteries.
From www.vox.com
Millions of used electric car batteries will help store energy for the grid. Maybe. Vox Electrodes Used In Car Batteries At the most basic level,. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an. Electrodes Used In Car Batteries.
From electronics360.globalspec.com
New Electrolyte for Lithium Batteries Can Extend Electric Car’s Driving Range Electronics360 Electrodes Used In Car Batteries This energy leaves the battery. At the most basic level,. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes,. Electrodes Used In Car Batteries.
From inside.lgensol.com
(Infographics 3) Battery Making at a Glance Battery LAB Electrodes Used In Car Batteries One way to understand electrochemical cells is to consider them in two commonly used items: The positive anode tends to be made up of. At the most basic level,. This energy leaves the battery. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. Electric car batteries rely. Electrodes Used In Car Batteries.
From www.biolinscientific.com
Wettability of electrodes Electrode calendering in Liion batteries Electrodes Used In Car Batteries This energy leaves the battery. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. At. Electrodes Used In Car Batteries.
From www.askiitians.com
WORKING OF CAR BATTERIES!!! askIITians Blog One place for all updates on IIT JEE & Medical Exams Electrodes Used In Car Batteries In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. This energy leaves the battery. One. Electrodes Used In Car Batteries.
From www.dreamstime.com
Single Electrode Used Car Sparks. Stock Image Image of closeup, garage 35208367 Electrodes Used In Car Batteries Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. This energy leaves the battery. In a study published june 22 in nature energy, stanford researchers demonstrate how their. Electrodes Used In Car Batteries.
From techbros.in
Discipline Helps Thick Battery Electrodes Deal with Electrical Car Challenges techbros.in Electrodes Used In Car Batteries The positive anode tends to be made up of. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering. Electrodes Used In Car Batteries.
From www.autocar.co.uk
Carbon electrodes promise EV battery performance breakthrough Autocar Electrodes Used In Car Batteries The positive anode tends to be made up of. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. At the most basic level,. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology. Electrodes Used In Car Batteries.
From theengineeringmindset.com
How a Car Battery Works The Engineering Mindset Electrodes Used In Car Batteries Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. This energy leaves the battery. At. Electrodes Used In Car Batteries.
From www.researchgate.net
Schematic illustration of a composite electrode in lithiumion batteries. Download Scientific Electrodes Used In Car Batteries At the most basic level,. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and. Electrodes Used In Car Batteries.
From www.electricity-magnetism.org
Batterie lithiumion Comment ça marche Réaction, anode et cathode Electrodes Used In Car Batteries At the most basic level,. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. The positive anode tends to be made up of. One way to understand electrochemical cells is to consider them in two commonly used items: Electric car batteries rely on the interplay of several. Electrodes Used In Car Batteries.
From saylordotorg.github.io
Electrochemistry Electrodes Used In Car Batteries The positive anode tends to be made up of. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. At the most basic level,. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology. Electrodes Used In Car Batteries.
From www.furukawa.co.jp
Lithiumion batteries Automotive (EV related parts) sector Application Industrial Laser Electrodes Used In Car Batteries The positive anode tends to be made up of. At the most basic level,. One way to understand electrochemical cells is to consider them in two commonly used items: Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. This energy leaves the battery. In a study published june 22 in. Electrodes Used In Car Batteries.
From www.pv-magazine.com
Hybrid electrodes for cheaper redox flow batteries pv magazine International Electrodes Used In Car Batteries In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. This energy leaves the battery. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and. Electrodes Used In Car Batteries.
From www.youtube.com
Extract Graphite electrode (Carbon rod) from battery. YouTube Electrodes Used In Car Batteries The positive anode tends to be made up of. This energy leaves the battery. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. One way to understand electrochemical cells is to consider them in two commonly used items: In general, a battery cell is made up of an anode, cathode,. Electrodes Used In Car Batteries.
From www.aquametals.com
What Are Battery Anode and Cathode Materials? AquaMetals Electrodes Used In Car Batteries Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. At the most basic level,. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. In a study published june 22 in nature energy, stanford researchers demonstrate how their. Electrodes Used In Car Batteries.
From www.azonano.com
Scientists Use Graphite Electrodes to Advance Rechargeable Aluminium Batteries Electrodes Used In Car Batteries In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries, a promising technology for powering electric vehicles, laptops and other devices. One way to understand electrochemical cells is to consider them in two commonly used items: Electric car batteries rely on the interplay of several key. Electrodes Used In Car Batteries.
From www.researchgate.net
Typical processes of battery electrode production Download Scientific Diagram Electrodes Used In Car Batteries This energy leaves the battery. The positive anode tends to be made up of. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium metal batteries,. Electrodes Used In Car Batteries.
From www.doitpoms.ac.uk
Lead/acid batteries Electrodes Used In Car Batteries Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. At the most basic level,. The positive anode tends to be made up of. This energy leaves the battery. In a study published june 22 in nature energy, stanford researchers demonstrate how their novel electrolyte design boosts the performance of lithium. Electrodes Used In Car Batteries.
From theconversation.com
Electric vehicle batteries what will they look like in the future? Electrodes Used In Car Batteries At the most basic level,. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. The positive anode tends to be made up of. Electric car batteries rely on the interplay of several key components, including electrolytes and electrodes, in order to function. In a study published june. Electrodes Used In Car Batteries.
From www.yourmechanic.com
How to Check Electrolyte Levels in Your Battery YourMechanic Advice Electrodes Used In Car Batteries This energy leaves the battery. At the most basic level,. In general, a battery cell is made up of an anode, cathode, separator and electrolyte which are packaged into an aluminium case. The positive anode tends to be made up of. One way to understand electrochemical cells is to consider them in two commonly used items: In a study published. Electrodes Used In Car Batteries.