Titration Lab With Citric Acid . In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). It is the purpose of this experiment to determine the. Some citrus fruits taste more sour, and therefore are more acidic, than others. Refer to part 1 for the background information and the titration method. The acid and the base react with one another according to the. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. 10 rows citric acid and sodium hydroxide are used for all three presented titrations. In this experiment you will titrate a measured volume of citric acid (c6h8o7) with a solution of naoh of a known concentration. To determine the amount of citric acid present in fruit juices. This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. 3 naoh (aq) + h 3 c 6. A second article will suggest applications of the same experiment that are suitable for experienced titrators. Express the sample’s acidity as grams of citric acid, c 6 h 8 o 7, per 100 ml. Because citric acid is a triprotic weak acid, we first must determine if the phenolphthalein end point corresponds to the first, second, or third equivalence point. Citric acid’s ladder diagram is shown in figure 9.2.15 a.
from www.chegg.com
Express the sample’s acidity as grams of citric acid, c 6 h 8 o 7, per 100 ml. 10 rows citric acid and sodium hydroxide are used for all three presented titrations. This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. It is the purpose of this experiment to determine the. Refer to part 1 for the background information and the titration method. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). The acid and the base react with one another according to the. To determine the amount of citric acid present in fruit juices. A second article will suggest applications of the same experiment that are suitable for experienced titrators. Citric acid’s ladder diagram is shown in figure 9.2.15 a.
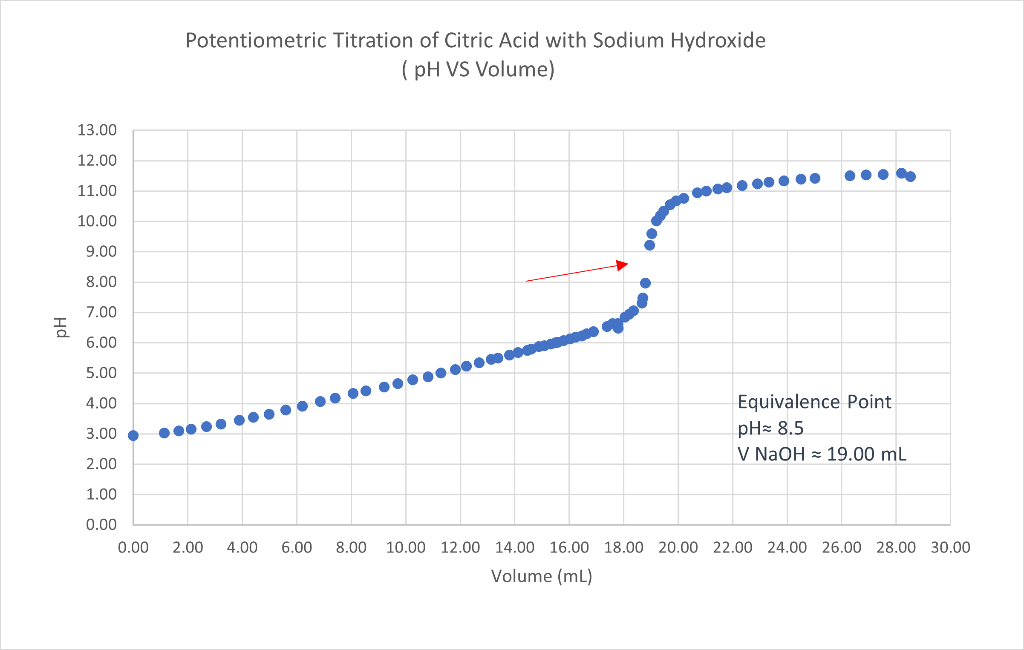
Solved Potentiometric Titration of Citric Acid with Sodium
Titration Lab With Citric Acid To determine the amount of citric acid present in fruit juices. To determine the amount of citric acid present in fruit juices. Some citrus fruits taste more sour, and therefore are more acidic, than others. Refer to part 1 for the background information and the titration method. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). 10 rows citric acid and sodium hydroxide are used for all three presented titrations. Citric acid’s ladder diagram is shown in figure 9.2.15 a. 3 naoh (aq) + h 3 c 6. Express the sample’s acidity as grams of citric acid, c 6 h 8 o 7, per 100 ml. This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. It is the purpose of this experiment to determine the. Because citric acid is a triprotic weak acid, we first must determine if the phenolphthalein end point corresponds to the first, second, or third equivalence point. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A second article will suggest applications of the same experiment that are suitable for experienced titrators. In this experiment you will titrate a measured volume of citric acid (c6h8o7) with a solution of naoh of a known concentration. The acid and the base react with one another according to the.
From www.youtube.com
Experiment for the Determination of amount of citric acid in lemon Titration Lab With Citric Acid 10 rows citric acid and sodium hydroxide are used for all three presented titrations. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Citric acid’s ladder diagram is shown in figure 9.2.15 a. In the case of the citric acid titration, a known amount of orange juice. Titration Lab With Citric Acid.
From ebinu.blog
AcidBase Titration Lab — DataClassroom / Acid Base Titration Titration Lab With Citric Acid Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A second article will suggest applications of the same experiment that are suitable for experienced titrators. Citric acid’s ladder diagram is shown in figure 9.2.15 a. It is the purpose of this experiment to determine the. 3 naoh. Titration Lab With Citric Acid.
From www.chegg.com
Solved EXPERIMENT 7 TITRATION OF CITRIC ACID IN GINGER ALE Titration Lab With Citric Acid Some citrus fruits taste more sour, and therefore are more acidic, than others. The acid and the base react with one another according to the. This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. Citric acid’s ladder diagram is shown in figure 9.2.15 a. A second article. Titration Lab With Citric Acid.
From www.researchgate.net
Titration curves of citric acid solution in (a) water and (b,c) in the Titration Lab With Citric Acid 3 naoh (aq) + h 3 c 6. It is the purpose of this experiment to determine the. A second article will suggest applications of the same experiment that are suitable for experienced titrators. To determine the amount of citric acid present in fruit juices. Be able to determine the k a or k b from ph data associated with. Titration Lab With Citric Acid.
From mungfali.com
Acid Base Titration Calculation Titration Lab With Citric Acid To determine the amount of citric acid present in fruit juices. A second article will suggest applications of the same experiment that are suitable for experienced titrators. It is the purpose of this experiment to determine the. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator. Titration Lab With Citric Acid.
From www.youtube.com
Fund Chem 2 Lab 10 Citric Acid Titration YouTube Titration Lab With Citric Acid Refer to part 1 for the background information and the titration method. The acid and the base react with one another according to the. Express the sample’s acidity as grams of citric acid, c 6 h 8 o 7, per 100 ml. To determine the amount of citric acid present in fruit juices. Some citrus fruits taste more sour, and. Titration Lab With Citric Acid.
From www.thinkswap.com
Citric Acid Titration Practical Chemistry Year 12 SACE Thinkswap Titration Lab With Citric Acid To determine the amount of citric acid present in fruit juices. Citric acid’s ladder diagram is shown in figure 9.2.15 a. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Express the sample’s acidity as grams of citric acid, c 6 h 8 o 7, per 100. Titration Lab With Citric Acid.
From www.chegg.com
Solved Consider the following titration of Citric Acid with Titration Lab With Citric Acid A second article will suggest applications of the same experiment that are suitable for experienced titrators. Express the sample’s acidity as grams of citric acid, c 6 h 8 o 7, per 100 ml. Because citric acid is a triprotic weak acid, we first must determine if the phenolphthalein end point corresponds to the first, second, or third equivalence point.. Titration Lab With Citric Acid.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Lab With Citric Acid 3 naoh (aq) + h 3 c 6. To determine the amount of citric acid present in fruit juices. It is the purpose of this experiment to determine the. 10 rows citric acid and sodium hydroxide are used for all three presented titrations. Some citrus fruits taste more sour, and therefore are more acidic, than others. Citric acid’s ladder diagram. Titration Lab With Citric Acid.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Lab With Citric Acid Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. Refer to part 1 for the background information and the titration method. In the case of. Titration Lab With Citric Acid.
From www.alamy.com
Titration experiment. Student adding an alkaline solution from a Titration Lab With Citric Acid The acid and the base react with one another according to the. In this experiment you will titrate a measured volume of citric acid (c6h8o7) with a solution of naoh of a known concentration. Express the sample’s acidity as grams of citric acid, c 6 h 8 o 7, per 100 ml. 10 rows citric acid and sodium hydroxide are. Titration Lab With Citric Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Lab With Citric Acid Because citric acid is a triprotic weak acid, we first must determine if the phenolphthalein end point corresponds to the first, second, or third equivalence point. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). Be able to determine the k. Titration Lab With Citric Acid.
From www.studocu.com
Molar Mass of Citric Acid Using Titration Lab write up Riley Titration Lab With Citric Acid In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). It is the purpose of this experiment to determine the. Citric acid’s ladder diagram is shown in figure 9.2.15 a. Because citric acid is a triprotic weak acid, we first must determine. Titration Lab With Citric Acid.
From uwaterloo.ca
Acidbase titrations with citric acid part 1 Chem 13 News Magazine Titration Lab With Citric Acid In this experiment you will titrate a measured volume of citric acid (c6h8o7) with a solution of naoh of a known concentration. Refer to part 1 for the background information and the titration method. Some citrus fruits taste more sour, and therefore are more acidic, than others. It is the purpose of this experiment to determine the. This article presents. Titration Lab With Citric Acid.
From www.youtube.com
Unit 11 Titration lab with citric acid and NaOH YouTube Titration Lab With Citric Acid In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. A second article will suggest applications of the same experiment. Titration Lab With Citric Acid.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Lab With Citric Acid Because citric acid is a triprotic weak acid, we first must determine if the phenolphthalein end point corresponds to the first, second, or third equivalence point. 3 naoh (aq) + h 3 c 6. To determine the amount of citric acid present in fruit juices. In this experiment you will titrate a measured volume of citric acid (c6h8o7) with a. Titration Lab With Citric Acid.
From www.scribd.com
Lab 3 Titration of Soda Citric Acid PDF Molar Concentration Acid Titration Lab With Citric Acid A second article will suggest applications of the same experiment that are suitable for experienced titrators. 10 rows citric acid and sodium hydroxide are used for all three presented titrations. Some citrus fruits taste more sour, and therefore are more acidic, than others. The acid and the base react with one another according to the. It is the purpose of. Titration Lab With Citric Acid.
From www.scribd.com
Citric Lab Experiment Titration Sodium Hydroxide Titration Lab With Citric Acid A second article will suggest applications of the same experiment that are suitable for experienced titrators. Some citrus fruits taste more sour, and therefore are more acidic, than others. Citric acid’s ladder diagram is shown in figure 9.2.15 a. Refer to part 1 for the background information and the titration method. Express the sample’s acidity as grams of citric acid,. Titration Lab With Citric Acid.
From studylib.net
Titration of Citric Acid East Stroudsburg University Titration Lab With Citric Acid 3 naoh (aq) + h 3 c 6. 10 rows citric acid and sodium hydroxide are used for all three presented titrations. It is the purpose of this experiment to determine the. This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. To determine the amount of citric. Titration Lab With Citric Acid.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific Titration Lab With Citric Acid This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. Citric acid’s ladder diagram is shown in figure 9.2.15 a. Because citric acid is a triprotic weak acid, we first must determine if the phenolphthalein end point corresponds to the first, second, or third equivalence point. Some citrus. Titration Lab With Citric Acid.
From www.studocu.com
AcidBase Titration Lab Part II Determination of Citric Acid in Titration Lab With Citric Acid In this experiment you will titrate a measured volume of citric acid (c6h8o7) with a solution of naoh of a known concentration. The acid and the base react with one another according to the. A second article will suggest applications of the same experiment that are suitable for experienced titrators. Citric acid’s ladder diagram is shown in figure 9.2.15 a.. Titration Lab With Citric Acid.
From www.chegg.com
Solved Potentiometric Titration of Citric Acid with Sodium Titration Lab With Citric Acid This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. To determine the amount of citric acid present in fruit juices. Refer to part 1 for the background information and the titration method. A second article will suggest applications of the same experiment that are suitable for experienced. Titration Lab With Citric Acid.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Lab With Citric Acid Because citric acid is a triprotic weak acid, we first must determine if the phenolphthalein end point corresponds to the first, second, or third equivalence point. 10 rows citric acid and sodium hydroxide are used for all three presented titrations. Be able to determine the k a or k b from ph data associated with the titration of a weak. Titration Lab With Citric Acid.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Titration Lab With Citric Acid The acid and the base react with one another according to the. Some citrus fruits taste more sour, and therefore are more acidic, than others. 10 rows citric acid and sodium hydroxide are used for all three presented titrations. In this experiment you will titrate a measured volume of citric acid (c6h8o7) with a solution of naoh of a known. Titration Lab With Citric Acid.
From www.chegg.com
Solved 9. Titration Curve for Citric Acid Clearly draw the Titration Lab With Citric Acid Some citrus fruits taste more sour, and therefore are more acidic, than others. This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. 3 naoh (aq) + h 3 c 6. The acid and the base react with one another according to the. It is the purpose of. Titration Lab With Citric Acid.
From studylib.net
Titration Lab How Much Citric Acid is in Your Soda? Titration Lab With Citric Acid 3 naoh (aq) + h 3 c 6. To determine the amount of citric acid present in fruit juices. A second article will suggest applications of the same experiment that are suitable for experienced titrators. The acid and the base react with one another according to the. Citric acid’s ladder diagram is shown in figure 9.2.15 a. In this experiment. Titration Lab With Citric Acid.
From mavink.com
Acid Base Titration Lab Procedure Titration Lab With Citric Acid 3 naoh (aq) + h 3 c 6. Refer to part 1 for the background information and the titration method. 10 rows citric acid and sodium hydroxide are used for all three presented titrations. The acid and the base react with one another according to the. To determine the amount of citric acid present in fruit juices. Be able to. Titration Lab With Citric Acid.
From www.studocu.com
Citric acid Estimation by Titration method ESTIMATION OF CITRIC ACID Titration Lab With Citric Acid It is the purpose of this experiment to determine the. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Refer to part 1 for the background information and the titration method. In the case of the citric acid titration, a known amount of orange juice is measured. Titration Lab With Citric Acid.
From www.scribd.com
Titration of Citric Acid CHEM 103 Lab PDF Titration Chemistry Titration Lab With Citric Acid Because citric acid is a triprotic weak acid, we first must determine if the phenolphthalein end point corresponds to the first, second, or third equivalence point. The acid and the base react with one another according to the. This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators.. Titration Lab With Citric Acid.
From www.visionlearning.com
Ácidos y Bases I Chemistry Visionlearning Titration Lab With Citric Acid This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. In this experiment you will titrate a measured volume of citric acid (c6h8o7) with a solution of naoh of a known concentration. 3 naoh (aq) + h 3 c 6. The acid and the base react with one. Titration Lab With Citric Acid.
From technologyinscience.blogspot.com
BioResource Citric Acid Production by Aspergillus niger, Estimation Titration Lab With Citric Acid Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A second article will suggest applications of the same experiment that are suitable for experienced titrators. Because citric acid is a triprotic weak acid, we first must determine if the phenolphthalein end point corresponds to the first, second,. Titration Lab With Citric Acid.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration Lab With Citric Acid The acid and the base react with one another according to the. 3 naoh (aq) + h 3 c 6. To determine the amount of citric acid present in fruit juices. Citric acid’s ladder diagram is shown in figure 9.2.15 a. Refer to part 1 for the background information and the titration method. Express the sample’s acidity as grams of. Titration Lab With Citric Acid.
From www.researchgate.net
Potentiometric titration curves of free citric acid and Cr 3þcitric Titration Lab With Citric Acid Refer to part 1 for the background information and the titration method. The acid and the base react with one another according to the. A second article will suggest applications of the same experiment that are suitable for experienced titrators. To determine the amount of citric acid present in fruit juices. It is the purpose of this experiment to determine. Titration Lab With Citric Acid.
From dxolbdanx.blob.core.windows.net
What Is A Titration For Dummies at Jimmie Cook blog Titration Lab With Citric Acid 3 naoh (aq) + h 3 c 6. Citric acid’s ladder diagram is shown in figure 9.2.15 a. Refer to part 1 for the background information and the titration method. In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). Some citrus. Titration Lab With Citric Acid.
From www.chegg.com
Solved EXPERIMENT 7 TITRATION OF CITRIC ACID IN GINGER ALE Titration Lab With Citric Acid In the case of the citric acid titration, a known amount of orange juice is measured into an erlenmeyer flask with an indicator solution containing phenolphthalein (the indicator). This article presents a neutralization titration of a citric acid solution by sodium hydroxide solution in a format suitable for beginner titrators. Because citric acid is a triprotic weak acid, we first. Titration Lab With Citric Acid.