Acetic Acid And Water . one example is the reaction of acetic acid with ammonia: for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. when acetic acid is dissolved in water there is an equilibrium reaction:
from www.chegg.com
one example is the reaction of acetic acid with ammonia: Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. when acetic acid is dissolved in water there is an equilibrium reaction: for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane.
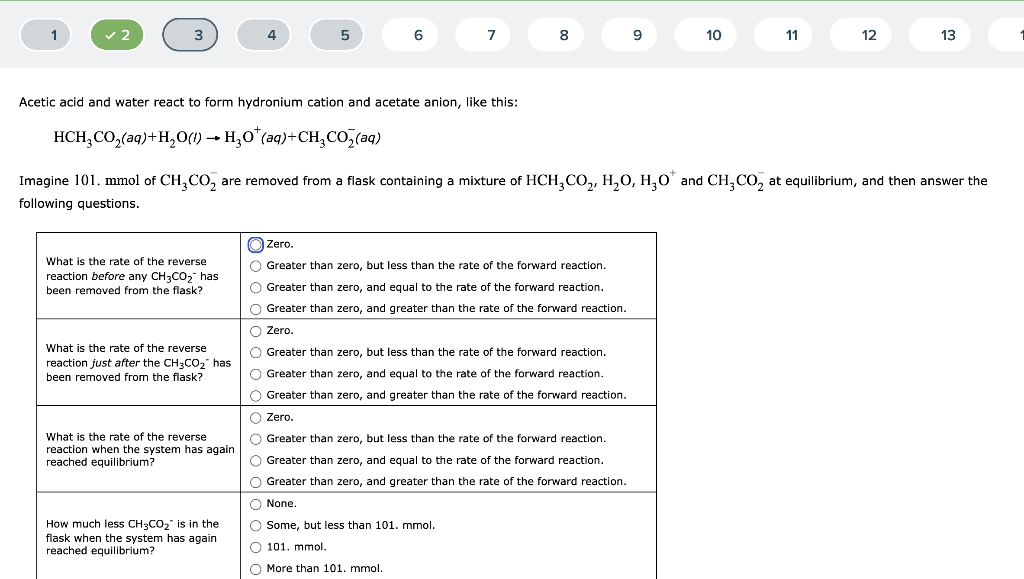
Solved Acetic acid and water react to form hydronium cation
Acetic Acid And Water 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. one example is the reaction of acetic acid with ammonia: when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. when acetic acid is dissolved in water there is an equilibrium reaction:
From demonstrations.wolfram.com
Extraction of Acetic Acid from Water Using Isopropyl Ether Wolfram Acetic Acid And Water when acetic acid is dissolved in water there is an equilibrium reaction: 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. for example, acetic acid (ch 3 cooh) is a weak acid,. Acetic Acid And Water.
From pubs.acs.org
Direct Photocatalytic Synthesis of Acetic Acid from Methane and CO at Acetic Acid And Water Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. one example is the reaction of acetic acid with ammonia: draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. when. Acetic Acid And Water.
From www.tessshebaylo.com
Write The Balanced Net Ionic Equation For Dissociation Of Acetic Acid Acetic Acid And Water for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. one example is the reaction of acetic acid with ammonia: draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. when. Acetic Acid And Water.
From people.cst.cmich.edu
Chapter 8 Phase Diagrams Acetic Acid And Water one example is the reaction of acetic acid with ammonia: Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. when acetic acid is dissolved in water there is an equilibrium reaction: 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. when acetic acid, ch3cooh, which is a. Acetic Acid And Water.
From www.numerade.com
SOLVED The esterification reaction of acetic acid with ethanol to give Acetic Acid And Water one example is the reaction of acetic acid with ammonia: when acetic acid is dissolved in water there is an equilibrium reaction: 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. for example, acetic acid (ch 3 cooh) is a weak acid, 1. Acetic Acid And Water.
From dxousamih.blob.core.windows.net
Acetic Acid Reaction With Water at Christian Pack blog Acetic Acid And Water Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. one example is the reaction of acetic acid with ammonia:. Acetic Acid And Water.
From www.chegg.com
Solved Acetic acid and water react to form hydronium cation Acetic Acid And Water one example is the reaction of acetic acid with ammonia: for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt.. Acetic Acid And Water.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Equation Tessshebaylo Acetic Acid And Water draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. Ch3co2h(aq)weakacid + nh3(aq)weakbase →. Acetic Acid And Water.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Acetic Acid And Water Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. one example is the reaction of acetic acid with ammonia: when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization. Acetic Acid And Water.
From www.researchgate.net
Phase diagram for acetic acid and water. 5 Download Scientific Diagram Acetic Acid And Water draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. one example is the reaction of acetic acid with ammonia: when. Acetic Acid And Water.
From separationprocesses.com
Figure072a Acetic Acid And Water one example is the reaction of acetic acid with ammonia: 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. when acetic acid is dissolved in water there is an equilibrium reaction: . Acetic Acid And Water.
From www.youtube.com
Ethanoic Acid + Water = ?? YouTube Acetic Acid And Water one example is the reaction of acetic acid with ammonia: when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. 13c and 1h nmr. Acetic Acid And Water.
From www.chegg.com
Solved Acetic acid and water react to form hydronium cation Acetic Acid And Water one example is the reaction of acetic acid with ammonia: 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules. Acetic Acid And Water.
From www.youtube.com
The Phase Diagram of a Three Component System Water, 1Butanol and Acetic Acid And Water 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. one example is the reaction of. Acetic Acid And Water.
From everythingdynamicequilibrium.weebly.com
Dissociation of Acetic Acid Dynamic Equilibrium Acetic Acid And Water when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. one example is the reaction of acetic acid with ammonia: for example, acetic acid (ch 3 cooh) is a weak acid,. Acetic Acid And Water.
From www.chegg.com
Solved An acetic acid molecule (CH3COOH) is shown Acetic Acid And Water for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. 13c and 1h nmr measurements to investigate the kinetics and the mechanism. Acetic Acid And Water.
From www.researchgate.net
Phase diagram for acetic acid and water. 5 Download Scientific Diagram Acetic Acid And Water when acetic acid is dissolved in water there is an equilibrium reaction: Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid. Acetic Acid And Water.
From www.youtube.com
Water , Acetic Acid and Chloroform system 01 PHASE RULE19 YouTube Acetic Acid And Water 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. one example is the reaction. Acetic Acid And Water.
From fr.wikidoc.org
Acetic acid wikidoc Acetic Acid And Water one example is the reaction of acetic acid with ammonia: for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. 13c and 1h nmr measurements to investigate the kinetics and the mechanism of. Acetic Acid And Water.
From www.pdfprof.com
methanol reacts with acetic acid to form methyl acetate and water Acetic Acid And Water for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. 13c and 1h. Acetic Acid And Water.
From www.chegg.com
Solved Acetic acid and water react to from hydronium cation Acetic Acid And Water Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. when acetic acid is dissolved in water there is an equilibrium reaction: when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. for example, acetic acid. Acetic Acid And Water.
From hubpages.com
Complete Chemistry of Hydrolysis & Hydration. HubPages Acetic Acid And Water for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. one example is the reaction of acetic acid with ammonia: when acetic acid is dissolved in water there is an equilibrium reaction: draw the structures of methanol. Acetic Acid And Water.
From www.semanticscholar.org
[PDF] Acetic acid—water system thermodynamical correlation of vapor Acetic Acid And Water when acetic acid is dissolved in water there is an equilibrium reaction: draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. one example is the reaction of acetic acid with ammonia: when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. for example, acetic acid (ch 3 cooh) is a. Acetic Acid And Water.
From courses.lumenlearning.com
15.4 Physical Properties of Carboxylic Acids The Basics of General Acetic Acid And Water 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. when acetic acid, ch3cooh, which is. Acetic Acid And Water.
From www.chegg.com
Solved Acetic acid and water react to form hydronium cation Acetic Acid And Water one example is the reaction of acetic acid with ammonia: draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. when acetic acid is dissolved in water there is an equilibrium reaction: 13c and 1h nmr measurements to investigate the kinetics. Acetic Acid And Water.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Net Ionic Equation Tessshebaylo Acetic Acid And Water for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization. Acetic Acid And Water.
From www.chegg.com
Solved A gaseous mixture of acetic acid and water at 1atm Acetic Acid And Water for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. when acetic acid is dissolved in water there is an equilibrium reaction: one example is the reaction of acetic acid with ammonia: 13c and 1h nmr measurements. Acetic Acid And Water.
From www.vectorstock.com
Formula and model of acetic acid molecule Vector Image Acetic Acid And Water 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. when. Acetic Acid And Water.
From wisc.pb.unizin.org
4.2 Classifying Chemical Reactions Chemistry Acetic Acid And Water for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. when acetic acid is dissolved in water there is an equilibrium reaction: draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. 13c and 1h nmr. Acetic Acid And Water.
From www.researchgate.net
Phase diagram for acetic acid and water. 5 Download Scientific Diagram Acetic Acid And Water when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. when acetic acid is dissolved in water there is an equilibrium reaction: one example is the reaction of acetic acid with ammonia: for example, acetic acid (ch 3 cooh) is a. Acetic Acid And Water.
From betance86662.blogspot.com
Acetic Acid To Acetic Anhydride Betance86662 Acetic Acid And Water 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. when. Acetic Acid And Water.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Equation Tessshebaylo Acetic Acid And Water when acetic acid is dissolved in water there is an equilibrium reaction: for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. when acetic acid, ch3cooh, which is a weak acid, is mixed with water,. Ch3co2h(aq)weakacid + nh3(aq)weakbase. Acetic Acid And Water.
From www.researchgate.net
Fig. S9. The number of adsorbed molecules for formic/acetic acids and Acetic Acid And Water for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. one example is the reaction of acetic acid with ammonia: when acetic acid is dissolved in water there is an equilibrium reaction: . Acetic Acid And Water.
From www.chegg.com
Solved Acetic acid and water react to form hydronium cation Acetic Acid And Water when acetic acid is dissolved in water there is an equilibrium reaction: 13c and 1h nmr measurements to investigate the kinetics and the mechanism of acetic acid (ch3co2h) ionization as a model for organic. draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. when acetic acid, ch3cooh, which is a weak acid, is mixed. Acetic Acid And Water.
From www.youtube.com
ChloroformWaterAcetic Acid system Three component system Acetic Acid And Water draw the structures of methanol (\(\mathrm{ch}_{3}\mathrm{oh}\)), acetic acid (\(\mathrm{ch}_{3}\mathrm{cooh}\)), and methane. for example, acetic acid (ch 3 cooh) is a weak acid, 1 m acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the. Ch3co2h(aq)weakacid + nh3(aq)weakbase → ch3co2nh4(aq)salt. when acetic acid is dissolved in water there is an equilibrium reaction:. Acetic Acid And Water.