Formula Triangles Chemistry . A general formula to calculate the concentration in g/dm 3 is: Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. It helps us understand that if we know any two of these values for a substance, we can find the third. Calculate the amount of solute, in moles,. If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. The mass, number of moles, concentration or volume of a substance can be. We can visualise their relationship using a formula triangle. Concentration refers to the amount of solute there is in a specific volume of the solvent. The gram formula mass of a substance is known as the mass of one mole.
from www.alamy.com
Concentration refers to the amount of solute there is in a specific volume of the solvent. The gram formula mass of a substance is known as the mass of one mole. A general formula to calculate the concentration in g/dm 3 is: It helps us understand that if we know any two of these values for a substance, we can find the third. Calculate the amount of solute, in moles,. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. We can visualise their relationship using a formula triangle. The mass, number of moles, concentration or volume of a substance can be. If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times.
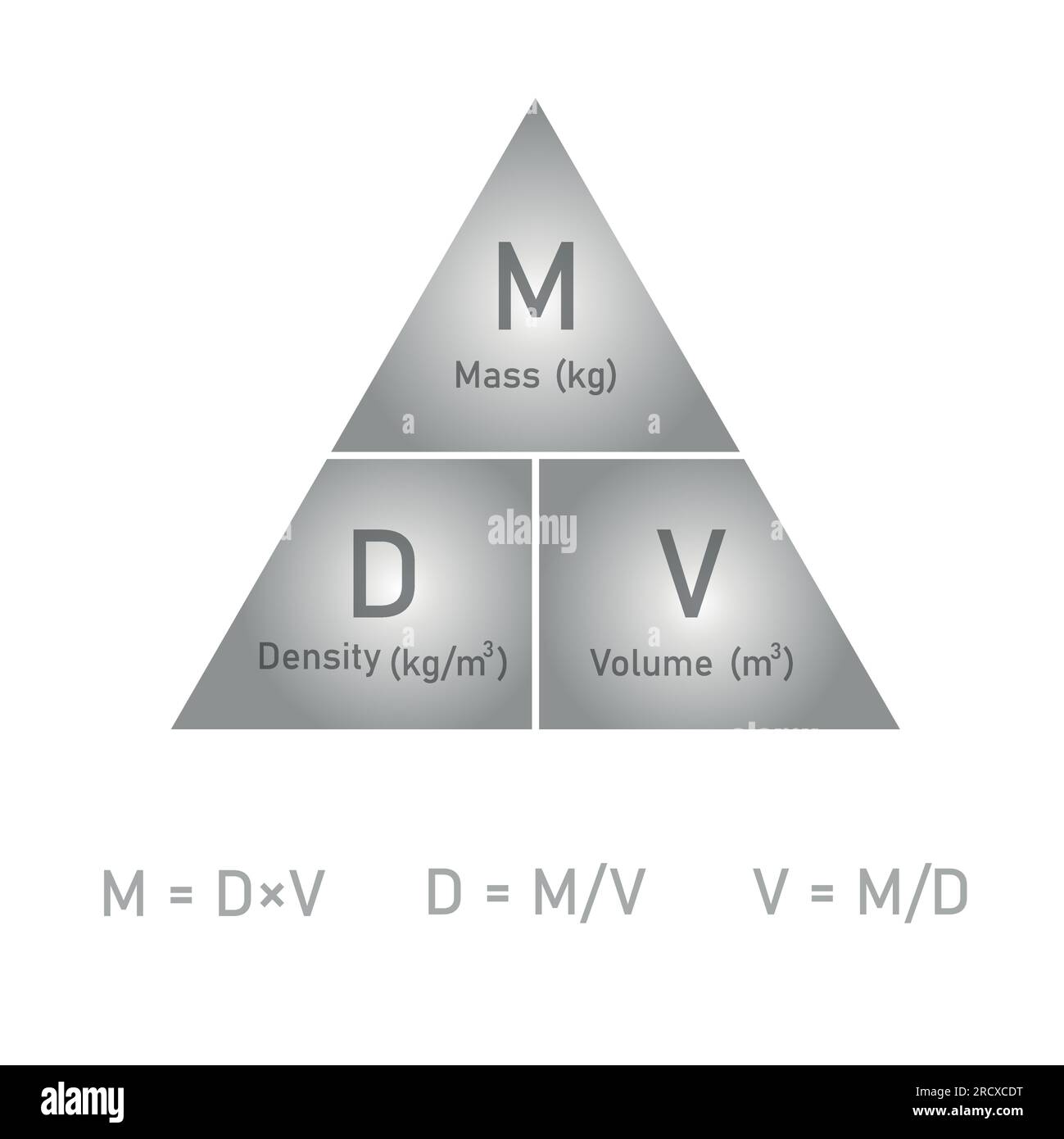
Density, mass and volume triangle formula in chemistry. Vector
Formula Triangles Chemistry It helps us understand that if we know any two of these values for a substance, we can find the third. The gram formula mass of a substance is known as the mass of one mole. Concentration refers to the amount of solute there is in a specific volume of the solvent. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. It helps us understand that if we know any two of these values for a substance, we can find the third. If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. The mass, number of moles, concentration or volume of a substance can be. We can visualise their relationship using a formula triangle. A general formula to calculate the concentration in g/dm 3 is: Calculate the amount of solute, in moles,.
From www.alamy.com
Density, mass and volume triangle formula in chemistry. Vector Formula Triangles Chemistry If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. It helps us understand that if we know any two of these values for a substance, we can find the third. The mass, number of moles,. Formula Triangles Chemistry.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Formula Triangles Chemistry Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. Concentration refers. Formula Triangles Chemistry.
From www.researchgate.net
The chemistry triangle. Download Scientific Diagram Formula Triangles Chemistry If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. Calculate the amount of solute, in moles,. We can visualise their relationship using a formula triangle. It helps us understand that if we know any two. Formula Triangles Chemistry.
From stock.adobe.com
The mole and concentration formula triangle or pyramid isolated on a Formula Triangles Chemistry Concentration refers to the amount of solute there is in a specific volume of the solvent. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. A general formula to calculate the concentration in g/dm 3 is: It helps us understand that if we know any. Formula Triangles Chemistry.
From www.alamy.com
Density, mass and volume triangle formula in chemistry. Vector Formula Triangles Chemistry Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. Concentration refers to the amount of solute there is in a specific volume of the solvent. A general formula to calculate the concentration in g/dm 3 is: If we know the mass of the substance and. Formula Triangles Chemistry.
From fity.club
Ch4 Molar Mass Formula Triangles Chemistry The mass, number of moles, concentration or volume of a substance can be. Concentration refers to the amount of solute there is in a specific volume of the solvent. It helps us understand that if we know any two of these values for a substance, we can find the third. Calculate the amount of solute, in moles,. If we know. Formula Triangles Chemistry.
From www.cuemath.com
Right Triangle Formulas Definition and Solved Examples Cuemath Formula Triangles Chemistry If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. Concentration refers to the amount of solute there is in a specific volume of the solvent. We can visualise their relationship using a formula triangle. It. Formula Triangles Chemistry.
From iteachly.com
Mole Conversion Worksheet and Activity ⋆ Formula Triangles Chemistry The gram formula mass of a substance is known as the mass of one mole. It helps us understand that if we know any two of these values for a substance, we can find the third. The mass, number of moles, concentration or volume of a substance can be. We can visualise their relationship using a formula triangle. If we. Formula Triangles Chemistry.
From www.youtube.com
Finding Moles by formula Cambridge IGCSE O level Chemistry 0620/0971 Formula Triangles Chemistry If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. It helps us understand that if we know any two of these values for a substance, we can find the third. The gram formula mass of. Formula Triangles Chemistry.
From ar.inspiredpencil.com
Triangle Chemistry Formula Triangles Chemistry If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. We can visualise their relationship using a formula triangle. A general formula to calculate the concentration in g/dm 3 is: Calculate the amount of solute, in. Formula Triangles Chemistry.
From www.alamyimages.fr
Conception scientifique du triangle de formule de Mole. Relation entre Formula Triangles Chemistry The gram formula mass of a substance is known as the mass of one mole. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. The mass, number of moles, concentration or volume of a substance can be. A general formula to calculate the concentration in. Formula Triangles Chemistry.
From www.yumpu.com
Formula Sheet (with Triangles) Formula Triangles Chemistry We can visualise their relationship using a formula triangle. If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. Calculate the amount of solute, in moles,. A general formula to calculate the concentration in g/dm 3. Formula Triangles Chemistry.
From syatillakmk.blogspot.com
SimplyChemistry C3 MOLEMASSNO.PARTICLE CONVERSIONS Formula Triangles Chemistry If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. A general formula to calculate the concentration in g/dm 3 is: Concentration refers to the amount of solute there is in a specific volume of the. Formula Triangles Chemistry.
From www.scribd.com
Mole Triangle PDF Formula Triangles Chemistry The mass, number of moles, concentration or volume of a substance can be. If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. Calculate the amount of solute, in moles,. It helps us understand that if. Formula Triangles Chemistry.
From www.alamy.com
Density, mass and volume triangle formula in chemistry. Vector Formula Triangles Chemistry Calculate the amount of solute, in moles,. A general formula to calculate the concentration in g/dm 3 is: The gram formula mass of a substance is known as the mass of one mole. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. The mass, number. Formula Triangles Chemistry.
From sites.google.com
2Mole Triangle Lufkin Chemistry Formula Triangles Chemistry It helps us understand that if we know any two of these values for a substance, we can find the third. We can visualise their relationship using a formula triangle. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. Concentration refers to the amount of. Formula Triangles Chemistry.
From www.slideserve.com
PPT Chemistry Calculations PowerPoint Presentation, free download Formula Triangles Chemistry We can visualise their relationship using a formula triangle. It helps us understand that if we know any two of these values for a substance, we can find the third. The mass, number of moles, concentration or volume of a substance can be. The gram formula mass of a substance is known as the mass of one mole. A general. Formula Triangles Chemistry.
From www.pinterest.com
GCSE Chemistry Formula Triangles Display Bunting Gcse chemistry Formula Triangles Chemistry Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. A general formula to calculate the concentration in g/dm 3 is: Concentration refers to the amount of solute there is in a specific volume of the solvent. Calculate the amount of solute, in moles,. We can. Formula Triangles Chemistry.
From www.thesciencehive.co.uk
Chemical formulae, equations and calculations GCSE — the science sauce Formula Triangles Chemistry The gram formula mass of a substance is known as the mass of one mole. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. Calculate the amount of solute, in moles,. A general formula to calculate the concentration in g/dm 3 is: The mass, number. Formula Triangles Chemistry.
From www.bajeczneobrazy.pl
The mole formula triangle or pyramid isolated on a white background Formula Triangles Chemistry Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. The gram formula mass of a substance is known as the mass of one mole. A general formula to calculate the concentration in g/dm 3 is: We can visualise their relationship using a formula triangle. Concentration. Formula Triangles Chemistry.
From www.tes.com
AS Chemistry The Mole and The Avogadro Constant Teaching Resources Formula Triangles Chemistry The mass, number of moles, concentration or volume of a substance can be. We can visualise their relationship using a formula triangle. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. Concentration refers to the amount of solute there is in a specific volume of. Formula Triangles Chemistry.
From www.alamy.com
Density, mass and volume triangle formula in chemistry. Vector Formula Triangles Chemistry A general formula to calculate the concentration in g/dm 3 is: Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by. Formula Triangles Chemistry.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.19 carry out mole calculations using Formula Triangles Chemistry Concentration refers to the amount of solute there is in a specific volume of the solvent. We can visualise their relationship using a formula triangle. If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. Calculate. Formula Triangles Chemistry.
From www.istockphoto.com
The Mole Formula Triangle Or Pyramid With Avogadro Number Or Avogadro Formula Triangles Chemistry It helps us understand that if we know any two of these values for a substance, we can find the third. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles, mass and volume at rtp. The mass, number of moles, concentration or volume of a substance can be. A general. Formula Triangles Chemistry.
From www.alamy.com
Density, mass and volume triangle formula in chemistry. Vector Formula Triangles Chemistry The mass, number of moles, concentration or volume of a substance can be. The gram formula mass of a substance is known as the mass of one mole. Concentration refers to the amount of solute there is in a specific volume of the solvent. If we know the mass of the substance and the molecular weight we can work out. Formula Triangles Chemistry.
From www.thesciencehive.co.uk
Rate of Reaction (AQA) — the science sauce Formula Triangles Chemistry If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. Concentration refers to the amount of solute there is in a specific volume of the solvent. Calculate the amount of solute, in moles,. A general formula. Formula Triangles Chemistry.
From chem.libretexts.org
Pascal’s Triangle Chemistry LibreTexts Formula Triangles Chemistry A general formula to calculate the concentration in g/dm 3 is: If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. We can visualise their relationship using a formula triangle. The gram formula mass of a. Formula Triangles Chemistry.
From www.alamy.com
Scientific Designing of The Mole And Molar Volume Formula Triangle Formula Triangles Chemistry The gram formula mass of a substance is known as the mass of one mole. If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. The mass, number of moles, concentration or volume of a substance. Formula Triangles Chemistry.
From www.savemyexams.com
Titration Calculations Edexcel GCSE Chemistry Revision Notes 2018 Formula Triangles Chemistry Calculate the amount of solute, in moles,. We can visualise their relationship using a formula triangle. The mass, number of moles, concentration or volume of a substance can be. Concentration refers to the amount of solute there is in a specific volume of the solvent. A general formula to calculate the concentration in g/dm 3 is: It helps us understand. Formula Triangles Chemistry.
From www.alamy.com
Density, mass and volume triangle formula in chemistry. Physics Formula Triangles Chemistry If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. Concentration refers to the amount of solute there is in a specific volume of the solvent. A general formula to calculate the concentration in g/dm 3. Formula Triangles Chemistry.
From www.pinterest.com
41ChemistryFormulaTrianglesLabAnswers.docx Chemistry, Education Formula Triangles Chemistry The gram formula mass of a substance is known as the mass of one mole. A general formula to calculate the concentration in g/dm 3 is: Concentration refers to the amount of solute there is in a specific volume of the solvent. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between. Formula Triangles Chemistry.
From www.tes.com
Equation Triangles Posters Teaching Resources Formula Triangles Chemistry A general formula to calculate the concentration in g/dm 3 is: We can visualise their relationship using a formula triangle. The mass, number of moles, concentration or volume of a substance can be. The gram formula mass of a substance is known as the mass of one mole. Calculate the amount of solute, in moles,. Concentration refers to the amount. Formula Triangles Chemistry.
From www.youtube.com
Combining Triangles National 5 Chemistry Lesson 8 YouTube Formula Triangles Chemistry It helps us understand that if we know any two of these values for a substance, we can find the third. Calculate the amount of solute, in moles,. The mass, number of moles, concentration or volume of a substance can be. Explore how to calculate gas volumes for igcse chemistry using formula triangles and avogadro's law, covering conversions between moles,. Formula Triangles Chemistry.
From www.youtube.com
Mass Triangle National 5 Chemistry Lesson 5 YouTube Formula Triangles Chemistry It helps us understand that if we know any two of these values for a substance, we can find the third. We can visualise their relationship using a formula triangle. Calculate the amount of solute, in moles,. Concentration refers to the amount of solute there is in a specific volume of the solvent. If we know the mass of the. Formula Triangles Chemistry.
From www.thesciencehive.co.uk
Chemical formulae, equations and calculations GCSE — the science sauce Formula Triangles Chemistry If we know the mass of the substance and the molecular weight we can work out how many moles of the substance we have by rearranging the formula \begin {equation} \text {molecular weight} \times. Concentration refers to the amount of solute there is in a specific volume of the solvent. The gram formula mass of a substance is known as. Formula Triangles Chemistry.