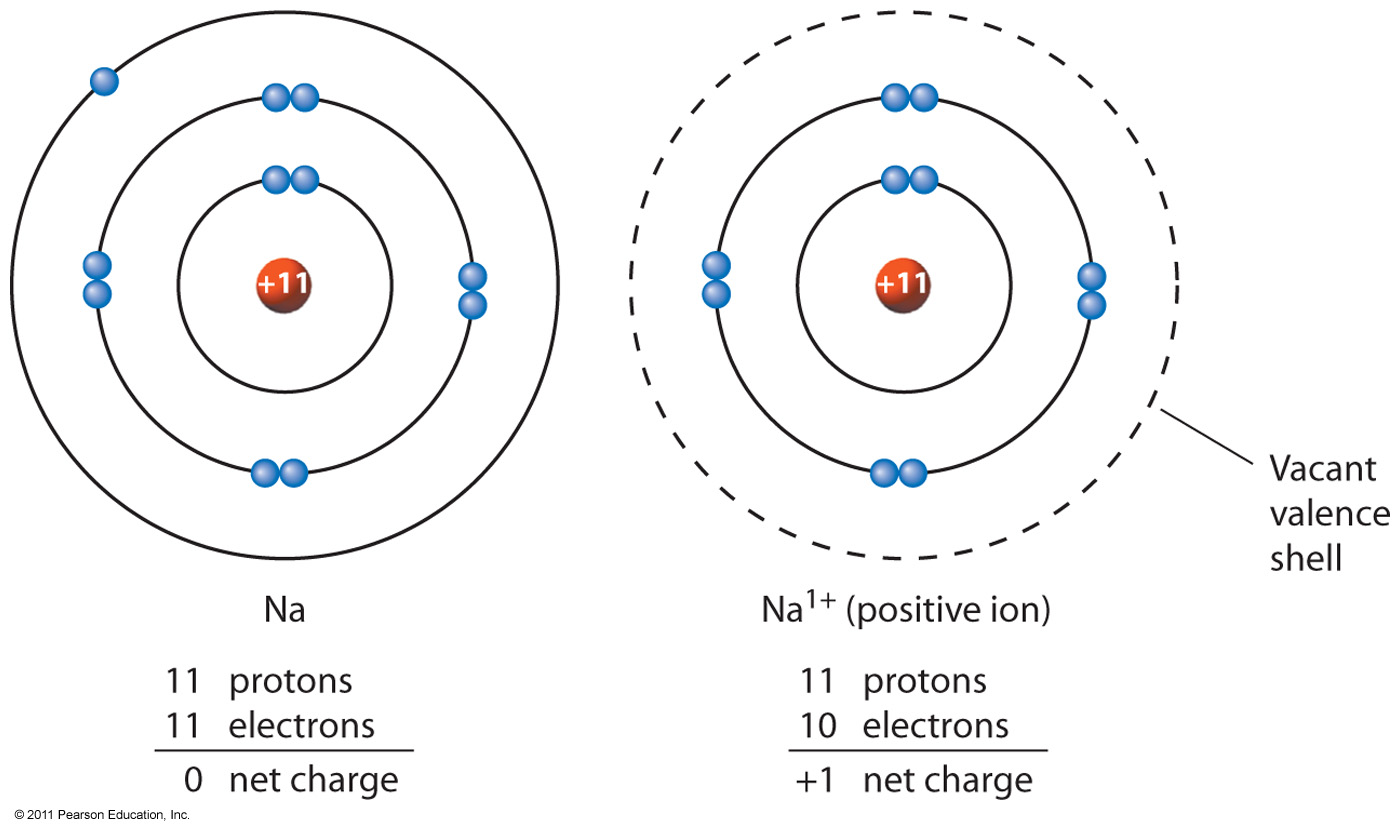
Iron Have A Charge . When atoms gain electron/s, the negatively charged ion is formed,. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). An atom of iron (fe) has 26 protons. You can use this table to predict. For example, iron( ii ) has a 2+ charge; 93 rows this table shows the most common charges for atoms of the chemical elements. This electric charge generated on the ion is known as ionic charge. Uncombined elements have an oxidation state of 0. A table with common ionic charges can also help determine oxidation states and chemical reactions. It is defined as being the charge that an atom would have if all bonds were ionic. This particular can have different numbers of electrons but we’ll say this example has 23 electrons.
from exosfhwgc.blob.core.windows.net
For example, iron( ii ) has a 2+ charge; This particular can have different numbers of electrons but we’ll say this example has 23 electrons. When atoms gain electron/s, the negatively charged ion is formed,. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). You can use this table to predict. A table with common ionic charges can also help determine oxidation states and chemical reactions. Uncombined elements have an oxidation state of 0. This electric charge generated on the ion is known as ionic charge. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows this table shows the most common charges for atoms of the chemical elements.
Iron Positive Charge at Henry Souza blog
Iron Have A Charge When atoms gain electron/s, the negatively charged ion is formed,. When atoms gain electron/s, the negatively charged ion is formed,. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. An atom of iron (fe) has 26 protons. Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of the chemical elements. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. It is defined as being the charge that an atom would have if all bonds were ionic. You can use this table to predict. A table with common ionic charges can also help determine oxidation states and chemical reactions. This electric charge generated on the ion is known as ionic charge. For example, iron( ii ) has a 2+ charge; For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide).
From www.flinnsci.ca
Ion Names, Formulas and Charges Chart Flinn Scientific Iron Have A Charge 93 rows this table shows the most common charges for atoms of the chemical elements. For example, iron( ii ) has a 2+ charge; You can use this table to predict. It is defined as being the charge that an atom would have if all bonds were ionic. For example, knowing that iron can have a +2 or +3 charge. Iron Have A Charge.
From www.nagwa.com
Question Video Identifying the Balanced Symbolic Equation for the Iron Have A Charge For example, iron( ii ) has a 2+ charge; It is defined as being the charge that an atom would have if all bonds were ionic. A table with common ionic charges can also help determine oxidation states and chemical reactions. 93 rows this table shows the most common charges for atoms of the chemical elements. Roman numeral notation indicates. Iron Have A Charge.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Iron Have A Charge 93 rows this table shows the most common charges for atoms of the chemical elements. When atoms gain electron/s, the negatively charged ion is formed,. For example, iron( ii ) has a 2+ charge; This particular can have different numbers of electrons but we’ll say this example has 23 electrons. For example, knowing that iron can have a +2 or. Iron Have A Charge.
From www.pinterest.jp
Element Charges Chart How to Know the Charge of an Atom How to know Iron Have A Charge An atom of iron (fe) has 26 protons. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows this table shows the most common charges for atoms of the chemical elements. A table with common ionic charges can also help determine oxidation states and chemical reactions. For example, knowing that iron. Iron Have A Charge.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Have A Charge Roman numeral notation indicates charge of ion when element commonly forms more than one ion. 93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table to predict. When atoms gain electron/s, the negatively charged ion is formed,. For example, knowing that iron can have a +2 or +3 charge can. Iron Have A Charge.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Iron (Fe Iron Have A Charge When atoms gain electron/s, the negatively charged ion is formed,. An atom of iron (fe) has 26 protons. A table with common ionic charges can also help determine oxidation states and chemical reactions. It is defined as being the charge that an atom would have if all bonds were ionic. For example, iron( ii ) has a 2+ charge; You. Iron Have A Charge.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID3048271 Iron Have A Charge You can use this table to predict. A table with common ionic charges can also help determine oxidation states and chemical reactions. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of the chemical elements.. Iron Have A Charge.
From www.slideserve.com
PPT Atoms and Ions PowerPoint Presentation, free download ID3945266 Iron Have A Charge It is defined as being the charge that an atom would have if all bonds were ionic. A table with common ionic charges can also help determine oxidation states and chemical reactions. When atoms gain electron/s, the negatively charged ion is formed,. An atom of iron (fe) has 26 protons. For example, iron( ii ) has a 2+ charge; For. Iron Have A Charge.
From gionamhtc.blob.core.windows.net
Iron Charge Of Ion at Steven Tritt blog Iron Have A Charge For example, iron( ii ) has a 2+ charge; You can use this table to predict. Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of the chemical elements. A table with common ionic charges can also help determine oxidation states and chemical reactions. It is defined as being the. Iron Have A Charge.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Have A Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). An atom of iron (fe) has 26 protons. It is defined as being the charge that an atom would have if all bonds were ionic. A table with. Iron Have A Charge.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Have A Charge When atoms gain electron/s, the negatively charged ion is formed,. Uncombined elements have an oxidation state of 0. For example, iron( ii ) has a 2+ charge; It is defined as being the charge that an atom would have if all bonds were ionic. An atom of iron (fe) has 26 protons. This electric charge generated on the ion is. Iron Have A Charge.
From www.youtube.com
How To Determine The Charge of Elements and Ions Chemistry YouTube Iron Have A Charge An atom of iron (fe) has 26 protons. 93 rows this table shows the most common charges for atoms of the chemical elements. When atoms gain electron/s, the negatively charged ion is formed,. This electric charge generated on the ion is known as ionic charge. For example, iron( ii ) has a 2+ charge; This particular can have different numbers. Iron Have A Charge.
From www.researchgate.net
(PDF) Study of the charge exchange process of iron impurity atoms in Iron Have A Charge For example, iron( ii ) has a 2+ charge; 93 rows this table shows the most common charges for atoms of the chemical elements. An atom of iron (fe) has 26 protons. A table with common ionic charges can also help determine oxidation states and chemical reactions. You can use this table to predict. Roman numeral notation indicates charge of. Iron Have A Charge.
From www.slideserve.com
PPT The Rusting of Iron PowerPoint Presentation, free download ID Iron Have A Charge This electric charge generated on the ion is known as ionic charge. When atoms gain electron/s, the negatively charged ion is formed,. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. 93 rows this table shows the most common charges for atoms of the chemical elements. For example, knowing that iron can have a. Iron Have A Charge.
From exosfhwgc.blob.core.windows.net
Iron Positive Charge at Henry Souza blog Iron Have A Charge For example, iron( ii ) has a 2+ charge; When atoms gain electron/s, the negatively charged ion is formed,. Uncombined elements have an oxidation state of 0. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. For example, knowing that iron can have a +2 or +3 charge can help predict the formation. Iron Have A Charge.
From www.youtube.com
Why metals have positive charge and Non metals carries negative charges Iron Have A Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). 93 rows this table shows the most common charges for atoms of the chemical elements. For example, iron( ii ) has a 2+ charge; An atom of iron. Iron Have A Charge.
From www.mdpi.com
Nanomaterials Free FullText Iron OxideMesoporous Silica Core Iron Have A Charge A table with common ionic charges can also help determine oxidation states and chemical reactions. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron. Iron Have A Charge.
From fyoxjsygg.blob.core.windows.net
Iron Electron Orbitals at Alexander Adams blog Iron Have A Charge Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). A table with common ionic charges can also help determine oxidation states and. Iron Have A Charge.
From klaxfvotc.blob.core.windows.net
What Is Irons Charge In Iron (Ii) Phosphate at Ha Lumpkin blog Iron Have A Charge This particular can have different numbers of electrons but we’ll say this example has 23 electrons. This electric charge generated on the ion is known as ionic charge. A table with common ionic charges can also help determine oxidation states and chemical reactions. An atom of iron (fe) has 26 protons. For example, iron( ii ) has a 2+ charge;. Iron Have A Charge.
From www.reddit.com
What does Marvel 8th mean? and why does Iron Man have charged? r Iron Have A Charge This particular can have different numbers of electrons but we’ll say this example has 23 electrons. An atom of iron (fe) has 26 protons. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). This electric charge generated. Iron Have A Charge.
From www.slideserve.com
PPT Transition Metals & Complex ions PowerPoint Presentation ID5713951 Iron Have A Charge You can use this table to predict. It is defined as being the charge that an atom would have if all bonds were ionic. When atoms gain electron/s, the negatively charged ion is formed,. This electric charge generated on the ion is known as ionic charge. Roman numeral notation indicates charge of ion when element commonly forms more than one. Iron Have A Charge.
From www.youtube.com
IRON MAN HAS 400 CHARGED IRON VS THOR YouTube Iron Have A Charge This particular can have different numbers of electrons but we’ll say this example has 23 electrons. This electric charge generated on the ion is known as ionic charge. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide).. Iron Have A Charge.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Have A Charge For example, iron( ii ) has a 2+ charge; This electric charge generated on the ion is known as ionic charge. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). When atoms gain electron/s, the negatively charged. Iron Have A Charge.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Have A Charge This particular can have different numbers of electrons but we’ll say this example has 23 electrons. Uncombined elements have an oxidation state of 0. This electric charge generated on the ion is known as ionic charge. For example, iron( ii ) has a 2+ charge; When atoms gain electron/s, the negatively charged ion is formed,. A table with common ionic. Iron Have A Charge.
From www.researchgate.net
Chargedischarge curves of the iron molten air batteries with and Iron Have A Charge You can use this table to predict. An atom of iron (fe) has 26 protons. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. Uncombined elements have an oxidation state of 0. For example, iron( ii ) has a 2+ charge; 93 rows this table shows the most common charges for atoms of. Iron Have A Charge.
From www.slideserve.com
PPT Red Blood Cells (Erythrocytes) PowerPoint Presentation, free Iron Have A Charge When atoms gain electron/s, the negatively charged ion is formed,. A table with common ionic charges can also help determine oxidation states and chemical reactions. An atom of iron (fe) has 26 protons. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. This electric charge generated on the ion is known as ionic. Iron Have A Charge.
From gionamhtc.blob.core.windows.net
Iron Charge Of Ion at Steven Tritt blog Iron Have A Charge For example, iron( ii ) has a 2+ charge; An atom of iron (fe) has 26 protons. When atoms gain electron/s, the negatively charged ion is formed,. You can use this table to predict. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. 93 rows this table shows the most common charges for. Iron Have A Charge.
From sciencenotes.org
Iron Facts Atomic Number 26 or Fe Iron Have A Charge A table with common ionic charges can also help determine oxidation states and chemical reactions. You can use this table to predict. It is defined as being the charge that an atom would have if all bonds were ionic. This electric charge generated on the ion is known as ionic charge. For example, knowing that iron can have a +2. Iron Have A Charge.
From giomtnkgb.blob.core.windows.net
Iron Charged Particles at Bradley Salcedo blog Iron Have A Charge Uncombined elements have an oxidation state of 0. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. An atom of iron (fe) has 26 protons. A table with common ionic charges can also help determine oxidation states and. Iron Have A Charge.
From circuitenginejoan.z21.web.core.windows.net
Iron Box Working Principle Iron Have A Charge When atoms gain electron/s, the negatively charged ion is formed,. An atom of iron (fe) has 26 protons. 93 rows this table shows the most common charges for atoms of the chemical elements. This electric charge generated on the ion is known as ionic charge. For example, iron( ii ) has a 2+ charge; For example, knowing that iron can. Iron Have A Charge.
From exosfhwgc.blob.core.windows.net
Iron Positive Charge at Henry Souza blog Iron Have A Charge You can use this table to predict. 93 rows this table shows the most common charges for atoms of the chemical elements. When atoms gain electron/s, the negatively charged ion is formed,. An atom of iron (fe) has 26 protons. For example, iron( ii ) has a 2+ charge; A table with common ionic charges can also help determine oxidation. Iron Have A Charge.
From www.vecteezy.com
charge word of iron on carbon 7617218 Stock Photo at Vecteezy Iron Have A Charge Uncombined elements have an oxidation state of 0. This electric charge generated on the ion is known as ionic charge. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. You can use this table to predict. A table with common ionic charges can also help determine oxidation states and chemical reactions. For example,. Iron Have A Charge.
From www.youtube.com
Ionic Charge for Iron (Fe) YouTube Iron Have A Charge 93 rows this table shows the most common charges for atoms of the chemical elements. An atom of iron (fe) has 26 protons. This particular can have different numbers of electrons but we’ll say this example has 23 electrons. A table with common ionic charges can also help determine oxidation states and chemical reactions. When atoms gain electron/s, the negatively. Iron Have A Charge.
From www.napervilleintegratedwellness.com
Functional Medicine Naperville Trace Element Iron Iron Have A Charge A table with common ionic charges can also help determine oxidation states and chemical reactions. When atoms gain electron/s, the negatively charged ion is formed,. It is defined as being the charge that an atom would have if all bonds were ionic. This electric charge generated on the ion is known as ionic charge. Roman numeral notation indicates charge of. Iron Have A Charge.
From www.researchgate.net
charge of iron nanoparticles. Notes (A) Neutrally charged Iron Have A Charge It is defined as being the charge that an atom would have if all bonds were ionic. For example, iron( ii ) has a 2+ charge; A table with common ionic charges can also help determine oxidation states and chemical reactions. An atom of iron (fe) has 26 protons. This particular can have different numbers of electrons but we’ll say. Iron Have A Charge.