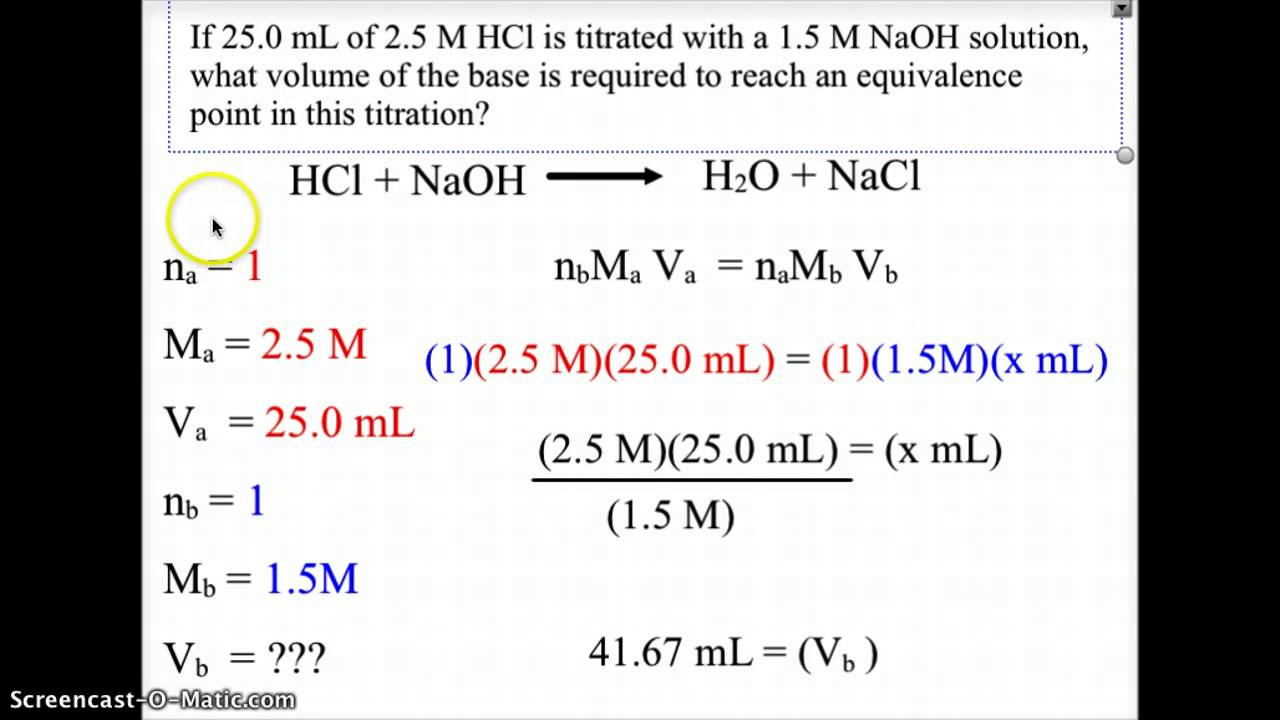
Titration Lab Balanced Equation . You can follow these 7. The equivalence point is the point in a neutralization reaction. Writing a balanced chemical equation. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. Titration between nitric acid and sodium carbonate is represented by the equation. The analyte (titrand) is the solution with an unknown. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base.
from www.youtube.com
The analyte (titrand) is the solution with an unknown. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Writing a balanced chemical equation. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Titration between nitric acid and sodium carbonate is represented by the equation. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. You can follow these 7. The equivalence point is the point in a neutralization reaction.
AcidBase Titration Equivalence Point YouTube
Titration Lab Balanced Equation The analyte (titrand) is the solution with an unknown. The equivalence point is the point in a neutralization reaction. Writing a balanced chemical equation. The analyte (titrand) is the solution with an unknown. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. Suppose that a titration is performed and \(20.70 \: Titration between nitric acid and sodium carbonate is represented by the equation. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. You can follow these 7. \ce{naoh}\) is required to reach the end point when titrated.
From www.youtube.com
Titration Stoichiometry HCl and Ba(OH)2 YouTube Titration Lab Balanced Equation The equivalence point is the point in a neutralization reaction. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. Titration between nitric acid and sodium carbonate is represented by the equation. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal. Titration Lab Balanced Equation.
From celhcddi.blob.core.windows.net
Titration Lab Math at Mike Brown blog Titration Lab Balanced Equation The analyte (titrand) is the solution with an unknown. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. The equivalence. Titration Lab Balanced Equation.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Lab Balanced Equation A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. Titration between nitric acid and sodium carbonate is represented by the equation. You can follow these 7. In a balanced neutralization equation,. Titration Lab Balanced Equation.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Titration Lab Balanced Equation Writing a balanced chemical equation. The analyte (titrand) is the solution with an unknown. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. The equivalence point is the point in a neutralization reaction. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2. Titration Lab Balanced Equation.
From www.chegg.com
Solved Titration Lab Balanced chemical equation for the Titration Lab Balanced Equation Suppose that a titration is performed and \(20.70 \: In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. The equivalence point is the point in a neutralization reaction. A titration is performed to determine the concentration of a reactant, this reactant. Titration Lab Balanced Equation.
From www.numerade.com
SOLVED Post LAB AcidBase Titration Name Dale Write the balanced Titration Lab Balanced Equation Writing a balanced chemical equation. The equivalence point is the point in a neutralization reaction. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. Suppose that a titration is performed and \(20.70 \: The analyte (titrand) is the solution with an unknown. Titration between nitric acid and sodium. Titration Lab Balanced Equation.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Lab Balanced Equation The analyte (titrand) is the solution with an unknown. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. Titration between nitric acid and sodium carbonate is represented by the equation. Suppose that a titration is performed and \(20.70 \: The equivalence point is the point in a neutralization reaction. In a balanced. Titration Lab Balanced Equation.
From www.studocu.com
Titration of diprotic acid lab Balanced equation 2 NaOH(aq) + H 2 A Titration Lab Balanced Equation The equivalence point is the point in a neutralization reaction. \ce{naoh}\) is required to reach the end point when titrated. Writing a balanced chemical equation. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. In a balanced neutralization equation, the moles of h + ions supplied by the. Titration Lab Balanced Equation.
From mavink.com
Titration Reaction Titration Lab Balanced Equation The analyte (titrand) is the solution with an unknown. You can follow these 7. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. Titration between nitric acid and sodium carbonate is represented by the equation. Writing a balanced chemical equation. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required. Titration Lab Balanced Equation.
From www.chegg.com
Solved Titrations Balanced equation is needed for every Titration Lab Balanced Equation In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. The equivalence point is the point in a neutralization reaction. \ce{naoh}\). Titration Lab Balanced Equation.
From www.savemyexams.com
Titrations OCR Gateway GCSE Chemistry Revision Notes 2018 Titration Lab Balanced Equation Titration between nitric acid and sodium carbonate is represented by the equation. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Suppose that a titration is performed and \(20.70 \: A titration is performed to determine the concentration of a reactant,. Titration Lab Balanced Equation.
From fity.club
Titration Calculations Titration Lab Balanced Equation 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. You can follow these 7. \ce{naoh}\) is required to reach the end point when titrated. Writing a balanced chemical equation. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. The analyte. Titration Lab Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Titration Lab Balanced Equation The analyte (titrand) is the solution with an unknown. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. Writing a balanced chemical equation. The equivalence point is the point in a neutralization reaction. Titration between nitric acid and sodium carbonate is represented by the equation. Suppose that a titration is performed and. Titration Lab Balanced Equation.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Lab Balanced Equation The analyte (titrand) is the solution with an unknown. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. Titration between. Titration Lab Balanced Equation.
From www.chegg.com
Solved S Titration Lab Balanced chemical equation for the Titration Lab Balanced Equation Titration between nitric acid and sodium carbonate is represented by the equation. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Suppose that a titration is performed and \(20.70 \: You can follow these 7. The analyte (titrand) is the solution. Titration Lab Balanced Equation.
From www.chegg.com
Solved Titration Lab Balanced chemical equation for the Titration Lab Balanced Equation The analyte (titrand) is the solution with an unknown. Suppose that a titration is performed and \(20.70 \: A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. Writing a balanced chemical equation. The equivalence point is the point in a neutralization reaction. Titration between nitric acid and sodium. Titration Lab Balanced Equation.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Lab Balanced Equation You can follow these 7. \ce{naoh}\) is required to reach the end point when titrated. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Suppose that a titration is performed and \(20.70 \: Writing a balanced chemical equation. Titration between nitric. Titration Lab Balanced Equation.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with Titration Lab Balanced Equation Writing a balanced chemical equation. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal. Titration Lab Balanced Equation.
From www.numerade.com
SOLVED CHEM 101 GENERAL CHEMISTRY Lab 7 AcidBase Titration (A Titration Lab Balanced Equation The analyte (titrand) is the solution with an unknown. \ce{naoh}\) is required to reach the end point when titrated. The equivalence point is the point in a neutralization reaction. Suppose that a titration is performed and \(20.70 \: In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh. Titration Lab Balanced Equation.
From joiswenze.blob.core.windows.net
Titration Volume Equation at Shirley Woodard blog Titration Lab Balanced Equation In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. Writing a balanced chemical equation. Suppose that a titration is performed. Titration Lab Balanced Equation.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Lab Balanced Equation The analyte (titrand) is the solution with an unknown. Suppose that a titration is performed and \(20.70 \: The equivalence point is the point in a neutralization reaction. Writing a balanced chemical equation. You can follow these 7. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. In a balanced neutralization equation,. Titration Lab Balanced Equation.
From dxopmiczw.blob.core.windows.net
How To Do Titration Calculations A Level Chemistry at Lorraine Nix blog Titration Lab Balanced Equation \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. Writing a balanced chemical equation. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the. Titration Lab Balanced Equation.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Lab Balanced Equation You can follow these 7. Suppose that a titration is performed and \(20.70 \: In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. \ce{naoh}\) is required to reach the end point when titrated. The equivalence point is the point in a. Titration Lab Balanced Equation.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Lab Balanced Equation In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. The analyte (titrand) is the solution with an unknown. Titration between nitric acid and sodium. Titration Lab Balanced Equation.
From www.slideserve.com
PPT Reactions in Aqueous Solutions II Calculations PowerPoint Titration Lab Balanced Equation The equivalence point is the point in a neutralization reaction. The analyte (titrand) is the solution with an unknown. A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. Suppose that a titration is performed and \(20.70 \: In a balanced neutralization equation, the moles of h + ions. Titration Lab Balanced Equation.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Titration Lab Balanced Equation You can follow these 7. \ce{naoh}\) is required to reach the end point when titrated. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Titration between nitric acid and sodium carbonate is represented by the equation. The equivalence point is the. Titration Lab Balanced Equation.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration Lab Balanced Equation \ce{naoh}\) is required to reach the end point when titrated. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Titration between nitric acid and sodium carbonate is represented by the equation. The equivalence point is the point in a neutralization reaction.. Titration Lab Balanced Equation.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Lab Balanced Equation You can follow these 7. Writing a balanced chemical equation. The analyte (titrand) is the solution with an unknown. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Titration between nitric acid and sodium carbonate is represented by the equation. 2h. Titration Lab Balanced Equation.
From unacademy.com
Mohr salt titration Titration Lab Balanced Equation A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. Writing a balanced chemical equation. \ce{naoh}\) is required to reach the end point when titrated. The analyte (titrand) is the solution with an unknown. Suppose that a titration is performed and \(20.70 \: You can follow these 7. Titration. Titration Lab Balanced Equation.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Lab Balanced Equation Suppose that a titration is performed and \(20.70 \: Titration between nitric acid and sodium carbonate is represented by the equation. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. The analyte (titrand) is the solution with an unknown. You can follow these 7. A titration is performed to determine the concentration. Titration Lab Balanced Equation.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Lab Balanced Equation \ce{naoh}\) is required to reach the end point when titrated. You can follow these 7. The equivalence point is the point in a neutralization reaction. Suppose that a titration is performed and \(20.70 \: In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by. Titration Lab Balanced Equation.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Lab Balanced Equation A titration is performed to determine the concentration of a reactant, this reactant may be either an acid or a base. The equivalence point is the point in a neutralization reaction. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Titration. Titration Lab Balanced Equation.
From studylib.net
Titration Lab Titration Lab Balanced Equation 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. Writing a balanced chemical equation. In a balanced neutralization equation, the moles of h + ions supplied by the acid is equal to the moles of oh − ions supplied by the base. Suppose that a titration is performed and \(20.70 \: A. Titration Lab Balanced Equation.
From studylib.net
CHEMISTRY I LAB INTRO TO TITRATION Titration Lab Balanced Equation You can follow these 7. \ce{naoh}\) is required to reach the end point when titrated. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. The analyte (titrand) is the solution with an unknown. Writing a balanced chemical equation. Titration between nitric acid and sodium carbonate is represented by the equation. A titration. Titration Lab Balanced Equation.
From www.numerade.com
A student carried out a titration using H2SO4 and KOH. The balanced Titration Lab Balanced Equation Suppose that a titration is performed and \(20.70 \: 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) + h 2o(l) + co2(g) 2 h n. Writing a balanced chemical equation. Titration between nitric acid and sodium carbonate is represented by the equation. You can follow these 7. \ce{naoh}\) is required to reach the end point when titrated. A titration. Titration Lab Balanced Equation.