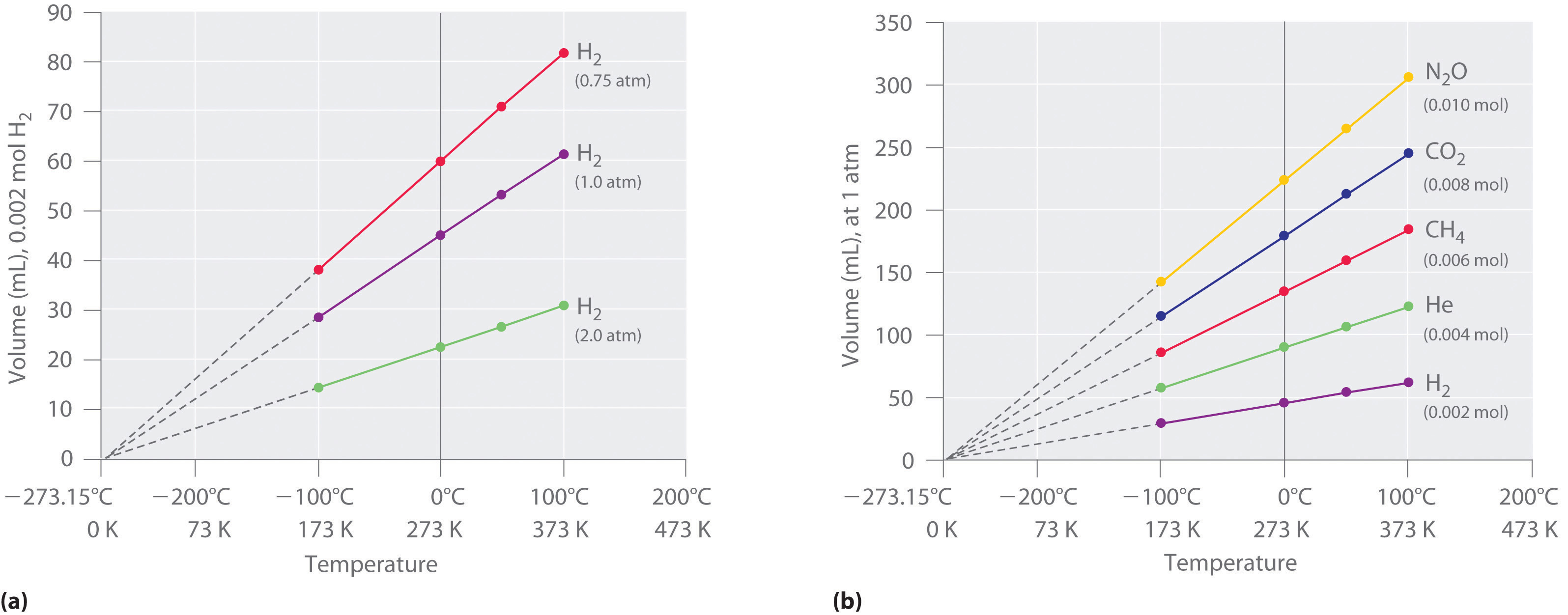
Absolute Pressure And Temperature . Absolute pressure is the sum of gauge pressure and atmospheric pressure. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. The absolute pressure measurement is required for. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: The ideal gas law states that. For reasons we will explore later, in most cases the absolute. P 1 t 1 = p 2 t 2. \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms.
from chem.libretexts.org
Absolute pressure is the sum of gauge pressure and atmospheric pressure. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: The ideal gas law states that. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. P 1 t 1 = p 2 t 2. The absolute pressure measurement is required for. Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. For reasons we will explore later, in most cases the absolute.
6.3 Relationships among Pressure, Temperature, Volume, and Amount Chemistry LibreTexts
Absolute Pressure And Temperature \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The absolute pressure measurement is required for. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. For reasons we will explore later, in most cases the absolute. Absolute pressure is the sum of gauge pressure and atmospheric pressure. P 1 t 1 = p 2 t 2. The ideal gas law states that. Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms.
From flatworldknowledge.lardbucket.org
Relationships among Pressure, Temperature, Volume, and Amount Absolute Pressure And Temperature P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. The absolute pressure measurement is required for. For reasons we will explore later, in most cases the absolute. Absolute pressure is the measure of pressure with respect. Absolute Pressure And Temperature.
From sites.google.com
Fluid statics Mr. Horwat's Science Pages Absolute Pressure And Temperature The absolute pressure measurement is required for. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. For reasons we will explore later, in most cases the absolute. P v = n r t {\displaystyle pv=nrt} where p is. Absolute Pressure And Temperature.
From ru.scribd.com
Pressure Temperature Chart Hvac Atmospheric Thermodynamics Absolute Pressure And Temperature P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: P 1 t 1 = p 2 t 2. Early scientists. Absolute Pressure And Temperature.
From www.embibe.com
Sketch a graph to show the relationship between the pressure of a gas and its absolute temperature Absolute Pressure And Temperature P 1 t 1 = p 2 t 2. \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms. The ideal gas law states that. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is. Absolute Pressure And Temperature.
From www.dreamstime.com
Types of Pressure Infographic Diagram Stock Vector Illustration of level, equation 245934725 Absolute Pressure And Temperature Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and. Absolute Pressure And Temperature.
From dokumen.tips
(PPTX) MECH1300 Basic Principles of Pneumatics Topics Absolute Pressure and Temperature Gas Laws Absolute Pressure And Temperature P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. \ [pv = nkt, \label {igl}\]. Absolute Pressure And Temperature.
From www.coursehero.com
[Solved] Need help with this one Question 2 At constant pressure, which... Course Hero Absolute Pressure And Temperature P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. The ideal gas law states that. P 1 t 1 = p 2 t 2. Absolute pressure is the measure of pressure with respect to absolute zero. Absolute Pressure And Temperature.
From sino-inst.com
Gauge Pressure vs. Absolute Pressure DifferencesRelationships Absolute Pressure And Temperature Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. P 1 t 1 = p 2 t 2. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the. Absolute Pressure And Temperature.
From www.slideserve.com
PPT Chapter 10 Gases & the Atmosphere PowerPoint Presentation ID611285 Absolute Pressure And Temperature For reasons we will explore later, in most cases the absolute. P 1 t 1 = p 2 t 2. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. The ideal gas law states that. Absolute pressure is. Absolute Pressure And Temperature.
From dept.swccd.edu
Lecture 16 Temperature and Theory Absolute Pressure And Temperature The absolute pressure measurement is required for. Absolute pressure is the sum of gauge pressure and atmospheric pressure. For reasons we will explore later, in most cases the absolute. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and.. Absolute Pressure And Temperature.
From www.numerade.com
A volume of 450 cm3 of air is measured at a pressure of 740 mm Hg absolute and a temperature of Absolute Pressure And Temperature Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. The ideal gas law states that. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute.. Absolute Pressure And Temperature.
From mungfali.com
Steam Pressure Chart Absolute Pressure And Temperature With the addition of avogadro's law, the combined gas law develops into the ideal gas law: Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r. Absolute Pressure And Temperature.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Chemistry LibreTexts Absolute Pressure And Temperature P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. The ideal gas law states that. Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum.. Absolute Pressure And Temperature.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor... Download Scientific Absolute Pressure And Temperature \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: The ideal gas law states that. Absolute pressure is the sum of gauge pressure. Absolute Pressure And Temperature.
From plot.ly
Graph showing Temperature Vs Absolute Pressure scatter chart made by Max.kerslake1 plotly Absolute Pressure And Temperature Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and. Absolute Pressure And Temperature.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an ideal gas. YouTube Absolute Pressure And Temperature Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. Absolute pressure is the sum of gauge pressure and atmospheric pressure. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: The absolute pressure measurement is required for. For reasons we will explore. Absolute Pressure And Temperature.
From ct-stem.northwestern.edu
CTSTEM Absolute Pressure And Temperature For reasons we will explore later, in most cases the absolute. \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms. The absolute pressure measurement is required for. Absolute pressure is the sum of gauge pressure and atmospheric pressure. With. Absolute Pressure And Temperature.
From www.researchgate.net
Relationships between the gas temperature, absolute humidity and water... Download Table Absolute Pressure And Temperature P 1 t 1 = p 2 t 2. \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms. For reasons we will explore later, in most cases the absolute. Absolute pressure is the measure of pressure with respect to. Absolute Pressure And Temperature.
From www.geeksforgeeks.org
Difference between Absolute Pressure and Atmospheric Pressure Absolute Pressure And Temperature The ideal gas law states that. P 1 t 1 = p 2 t 2. The absolute pressure measurement is required for. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it. Absolute Pressure And Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Absolute Pressure And Temperature The absolute pressure measurement is required for. P 1 t 1 = p 2 t 2. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: The ideal gas law states that. Absolute pressure is the sum of gauge pressure and atmospheric pressure. Absolute pressure is the measure of pressure with respect to absolute. Absolute Pressure And Temperature.
From www.savemyexams.co.uk
Gases & Absolute Temperature (2.1.5) CIE IGCSE Physics Revision Notes 2023 Save My Exams Absolute Pressure And Temperature For reasons we will explore later, in most cases the absolute. The ideal gas law states that. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. P 1 t 1 = p 2 t 2. Absolute. Absolute Pressure And Temperature.
From blog.beamex.com
Temperature units and temperature unit conversion Absolute Pressure And Temperature With the addition of avogadro's law, the combined gas law develops into the ideal gas law: P 1 t 1 = p 2 t 2. The absolute pressure measurement is required for. Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. Early scientists explored the relationships among the. Absolute Pressure And Temperature.
From socratic.org
Which graph shows the relationship between the temperature and volume of a gas according to Absolute Pressure And Temperature Absolute pressure is the sum of gauge pressure and atmospheric pressure. \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the. Absolute Pressure And Temperature.
From www.coyoteents.com
TEMPERATURE AND TIRE PRESSURE Coyote Enterprises Absolute Pressure And Temperature Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r. Absolute Pressure And Temperature.
From www.youtube.com
Absolute Pressure vs Gauge Pressure Fluid Mechanics Physics Problems YouTube Absolute Pressure And Temperature Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. P 1 t 1 = p 2 t 2. \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms.. Absolute Pressure And Temperature.
From www.sliderbase.com
Measurement of Pressure and Temperature Presentation Chemistry Absolute Pressure And Temperature For reasons we will explore later, in most cases the absolute. \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: Absolute pressure is. Absolute Pressure And Temperature.
From www.youtube.com
Absolute pressure, Gauge pressure, Atmospheric pressure Explained. Absolute pressure Gauge Absolute Pressure And Temperature With the addition of avogadro's law, the combined gas law develops into the ideal gas law: For reasons we will explore later, in most cases the absolute. The ideal gas law states that. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four. Absolute Pressure And Temperature.
From courses.lumenlearning.com
Phase Changes Physics Absolute Pressure And Temperature Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. The ideal gas law states that. For reasons we will explore later, in most cases the absolute. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles,. Absolute Pressure And Temperature.
From owlcation.com
The Theories and Behavior of Gas Owlcation Absolute Pressure And Temperature The absolute pressure measurement is required for. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: For reasons we will explore later, in most cases the absolute. P 1 t 1 = p 2 t 2. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume. Absolute Pressure And Temperature.
From www.youtube.com
Atmospheric Pressure, Gauge Pressure, Absolute Pressure and Units of Pressure Shubham Kola Absolute Pressure And Temperature Absolute pressure is the measure of pressure with respect to absolute zero pressure, which is the pressure of a perfect vacuum. P 1 t 1 = p 2 t 2. Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount. Absolute Pressure And Temperature.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID6307179 Absolute Pressure And Temperature Absolute pressure is the sum of gauge pressure and atmospheric pressure. The ideal gas law states that. The absolute pressure measurement is required for. For reasons we will explore later, in most cases the absolute. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: P 1 t 1 = p 2 t 2.. Absolute Pressure And Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Absolute Pressure And Temperature P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the. Absolute Pressure And Temperature.
From sites.google.com
E) Graphing semichem11ib Absolute Pressure And Temperature P 1 t 1 = p 2 t 2. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the number of moles, r is the universal gas constant and t is the absolute. The ideal gas law states that. Absolute pressure is the sum of gauge pressure and atmospheric pressure. \. Absolute Pressure And Temperature.
From siampressure.com
ทำความเข้าใจเกี่ยวกับ Absolute Pressure กันเถอะ Absolute Pressure And Temperature With the addition of avogadro's law, the combined gas law develops into the ideal gas law: Early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and. Absolute pressure is the measure of pressure with respect to absolute zero pressure,. Absolute Pressure And Temperature.
From www.engineeringtoolbox.com
Water Thermodynamic Properties Absolute Pressure And Temperature \ [pv = nkt, \label {igl}\] where \ (p\) is the absolute pressure of a gas, \ (v\) is the volume it occupies, \ (n\) is the number of atoms. With the addition of avogadro's law, the combined gas law develops into the ideal gas law: For reasons we will explore later, in most cases the absolute. The absolute pressure. Absolute Pressure And Temperature.