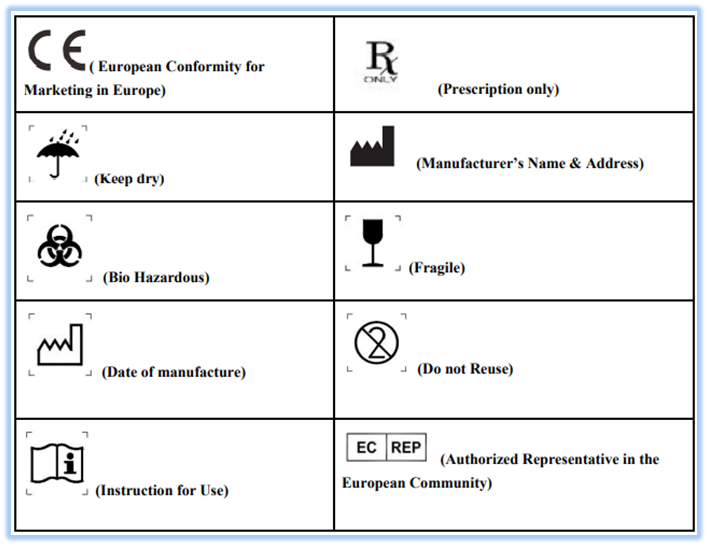
Australia Labeling Requirements Medical Devices . The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided with medical devices. Requirements to make australian medicine labels clearer and more consistent were introduced. Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. All medical devices supplied in australia must meet the relevant essential.
from knconsultingandservices.com
Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. All medical devices supplied in australia must meet the relevant essential. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided with medical devices. Requirements to make australian medicine labels clearer and more consistent were introduced.
What is Labelling? Medical Device Consulting Company
Australia Labeling Requirements Medical Devices The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided with medical devices. All medical devices supplied in australia must meet the relevant essential. Requirements to make australian medicine labels clearer and more consistent were introduced.
From www.regdesk.co
EFDA Guidance on Medical Device Labeling Special Requirements RegDesk Australia Labeling Requirements Medical Devices Australia's medicine labels are becoming clearer. All medical devices supplied in australia must meet the relevant essential. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided. Australia Labeling Requirements Medical Devices.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Australia Labeling Requirements Medical Devices The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided with medical devices. Requirements to make australian medicine labels clearer and more consistent were introduced. Australia's medicine labels are becoming clearer. All medical devices supplied in australia must meet the relevant essential. On monday (19 february,. Australia Labeling Requirements Medical Devices.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Presentation ID8427354 Australia Labeling Requirements Medical Devices The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Australia's medicine labels are becoming clearer. All medical devices supplied in australia must meet the relevant essential. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Requirements to make australian medicine. Australia Labeling Requirements Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Australia Labeling Requirements Medical Devices The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Australia's medicine labels are becoming clearer. Labelling and other information provided with medical devices. Requirements to make australian medicine labels clearer and. Australia Labeling Requirements Medical Devices.
From resources.sw.siemens.com
Labeling & UDI management for medical devices Australia Labeling Requirements Medical Devices Requirements to make australian medicine labels clearer and more consistent were introduced. All medical devices supplied in australia must meet the relevant essential. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided with medical devices. On monday (19 february, 2024) australia's therapeutic goods administration (tga). Australia Labeling Requirements Medical Devices.
From mavink.com
Medical Device Labeling Symbols Australia Labeling Requirements Medical Devices Australia's medicine labels are becoming clearer. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided with medical devices. Requirements to make australian medicine labels clearer and more consistent were introduced. All medical devices supplied in australia must meet the relevant essential. On monday (19 february,. Australia Labeling Requirements Medical Devices.
From medicaldevices.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Medical Devices Australia Labeling Requirements Medical Devices Requirements to make australian medicine labels clearer and more consistent were introduced. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. All medical devices supplied in australia must meet the relevant essential. Australia's medicine labels are becoming clearer. Labelling and other information provided with medical devices. The article provides an. Australia Labeling Requirements Medical Devices.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Australia Labeling Requirements Medical Devices All medical devices supplied in australia must meet the relevant essential. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided with medical devices. Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical. Australia Labeling Requirements Medical Devices.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Australia Labeling Requirements Medical Devices Labelling and other information provided with medical devices. All medical devices supplied in australia must meet the relevant essential. Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Requirements to make australian medicine labels clearer and more consistent were introduced. The article provides an. Australia Labeling Requirements Medical Devices.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Australia Labeling Requirements Medical Devices Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Requirements to make australian medicine labels clearer and more consistent were introduced. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. All medical devices supplied. Australia Labeling Requirements Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Australia Labeling Requirements Medical Devices The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Australia's medicine labels are becoming clearer. Labelling and other information provided with medical devices. All medical devices supplied in australia must meet. Australia Labeling Requirements Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Australia Labeling Requirements Medical Devices Requirements to make australian medicine labels clearer and more consistent were introduced. All medical devices supplied in australia must meet the relevant essential. Labelling and other information provided with medical devices. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Australia's medicine labels are becoming clearer. The article provides an. Australia Labeling Requirements Medical Devices.
From ambitiousmares.blogspot.com
31 Udi Label Examples Labels Design Ideas 2020 Australia Labeling Requirements Medical Devices Labelling and other information provided with medical devices. Australia's medicine labels are becoming clearer. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Requirements to make australian medicine labels clearer and. Australia Labeling Requirements Medical Devices.
From blog.globalvision.co
How to Ease the Labeling Proofreading Process for Medical Devices Australia Labeling Requirements Medical Devices Labelling and other information provided with medical devices. All medical devices supplied in australia must meet the relevant essential. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Australia's medicine labels. Australia Labeling Requirements Medical Devices.
From www.slideserve.com
PPT Medical Device Labeling Requirements VISTAAR PowerPoint Presentation ID8427354 Australia Labeling Requirements Medical Devices Labelling and other information provided with medical devices. Requirements to make australian medicine labels clearer and more consistent were introduced. All medical devices supplied in australia must meet the relevant essential. Australia's medicine labels are becoming clearer. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. On monday (19 february,. Australia Labeling Requirements Medical Devices.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Australia Labeling Requirements Medical Devices All medical devices supplied in australia must meet the relevant essential. Requirements to make australian medicine labels clearer and more consistent were introduced. Labelling and other information provided with medical devices. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Australia's medicine labels are becoming clearer. The article provides an. Australia Labeling Requirements Medical Devices.
From www.schlafenderhase.com
Medical Device Labeling Requirements Schlafender Hase Australia Labeling Requirements Medical Devices On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Australia's medicine labels are becoming clearer. Labelling and other information provided with medical devices. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Requirements to make australian medicine labels clearer and. Australia Labeling Requirements Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Australia Labeling Requirements Medical Devices The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Requirements to make australian medicine labels clearer and more consistent were introduced. Labelling and other information provided with medical devices. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Australia's medicine. Australia Labeling Requirements Medical Devices.
From mungfali.com
Medical Label Symbols Australia Labeling Requirements Medical Devices All medical devices supplied in australia must meet the relevant essential. Australia's medicine labels are becoming clearer. Requirements to make australian medicine labels clearer and more consistent were introduced. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated. Australia Labeling Requirements Medical Devices.
From coastlabel.com
Medical Device Labeling Medical Equipment Labels Coast Label Australia Labeling Requirements Medical Devices Australia's medicine labels are becoming clearer. Labelling and other information provided with medical devices. All medical devices supplied in australia must meet the relevant essential. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical. Australia Labeling Requirements Medical Devices.
From www.scribd.com
Requirements For Labelling of Medical Devices Mda PDF Medical Device Packaging And Labeling Australia Labeling Requirements Medical Devices Labelling and other information provided with medical devices. Australia's medicine labels are becoming clearer. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Requirements to make australian medicine labels clearer and more consistent were introduced. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the. Australia Labeling Requirements Medical Devices.
From nextplus.io
Medical Device Labeling Compliant & UserFriendly Guide Next Plus Australia Labeling Requirements Medical Devices Requirements to make australian medicine labels clearer and more consistent were introduced. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Australia's medicine labels are becoming clearer. Labelling and other information. Australia Labeling Requirements Medical Devices.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Australia Labeling Requirements Medical Devices Australia's medicine labels are becoming clearer. Labelling and other information provided with medical devices. All medical devices supplied in australia must meet the relevant essential. Requirements to make australian medicine labels clearer and more consistent were introduced. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. On monday (19 february,. Australia Labeling Requirements Medical Devices.
From www.scilife.io
Labeling Requirements for Medical Devices Scilife Australia Labeling Requirements Medical Devices All medical devices supplied in australia must meet the relevant essential. Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Requirements to make australian medicine labels clearer and more consistent were introduced. The article provides an overview of the legal framework for labeling requirements. Australia Labeling Requirements Medical Devices.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Registration & Labeling Australia Labeling Requirements Medical Devices On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Labelling and other information provided with medical devices. All medical devices supplied in australia must meet the relevant essential. Requirements to make australian medicine labels clearer and more consistent were introduced. Australia's medicine labels are becoming clearer. The article provides an. Australia Labeling Requirements Medical Devices.
From www.meddeviceonline.com
Infographic Medical Device Label Before And After EU MDR 10 Sticking Points Australia Labeling Requirements Medical Devices The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Labelling and other information provided with medical devices. Requirements to make australian medicine labels clearer and. Australia Labeling Requirements Medical Devices.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Australia Labeling Requirements Medical Devices Australia's medicine labels are becoming clearer. Labelling and other information provided with medical devices. Requirements to make australian medicine labels clearer and more consistent were introduced. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the. Australia Labeling Requirements Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Australia Labeling Requirements Medical Devices The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Requirements to make australian medicine labels clearer and more consistent were introduced. Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Labelling and other information. Australia Labeling Requirements Medical Devices.
From www.linkedin.com
7 Steps How to Get a CE Marking Certification for Medical Devices? Australia Labeling Requirements Medical Devices Requirements to make australian medicine labels clearer and more consistent were introduced. Australia's medicine labels are becoming clearer. All medical devices supplied in australia must meet the relevant essential. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided with medical devices. On monday (19 february,. Australia Labeling Requirements Medical Devices.
From dandelionsandthings.blogspot.com
31 Medical Device Label Requirements Label Design Ideas 2020 Australia Labeling Requirements Medical Devices Australia's medicine labels are becoming clearer. All medical devices supplied in australia must meet the relevant essential. Requirements to make australian medicine labels clearer and more consistent were introduced. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Labelling and other information provided with medical devices. The article provides an. Australia Labeling Requirements Medical Devices.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Australia Labeling Requirements Medical Devices Australia's medicine labels are becoming clearer. All medical devices supplied in australia must meet the relevant essential. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Labelling and other information provided. Australia Labeling Requirements Medical Devices.
From mavink.com
Medical Device Labeling Symbols Australia Labeling Requirements Medical Devices On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Requirements to make australian medicine labels clearer and more consistent were introduced. Labelling and other information provided with medical devices. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Australia's medicine. Australia Labeling Requirements Medical Devices.
From mavink.com
Medical Device Labeling Symbols Australia Labeling Requirements Medical Devices All medical devices supplied in australia must meet the relevant essential. Labelling and other information provided with medical devices. Australia's medicine labels are becoming clearer. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. Requirements to make australian medicine labels clearer and more consistent were introduced. The article provides an. Australia Labeling Requirements Medical Devices.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Australia Labeling Requirements Medical Devices Australia's medicine labels are becoming clearer. The article provides an overview of the legal framework for labeling requirements applicable to medical devices intended to be. Requirements to make australian medicine labels clearer and more consistent were introduced. Labelling and other information provided with medical devices. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the. Australia Labeling Requirements Medical Devices.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Australia Labeling Requirements Medical Devices All medical devices supplied in australia must meet the relevant essential. Labelling and other information provided with medical devices. Australia's medicine labels are becoming clearer. Requirements to make australian medicine labels clearer and more consistent were introduced. On monday (19 february, 2024) australia's therapeutic goods administration (tga) released updated guidance on the medical device labelling obligations. The article provides an. Australia Labeling Requirements Medical Devices.