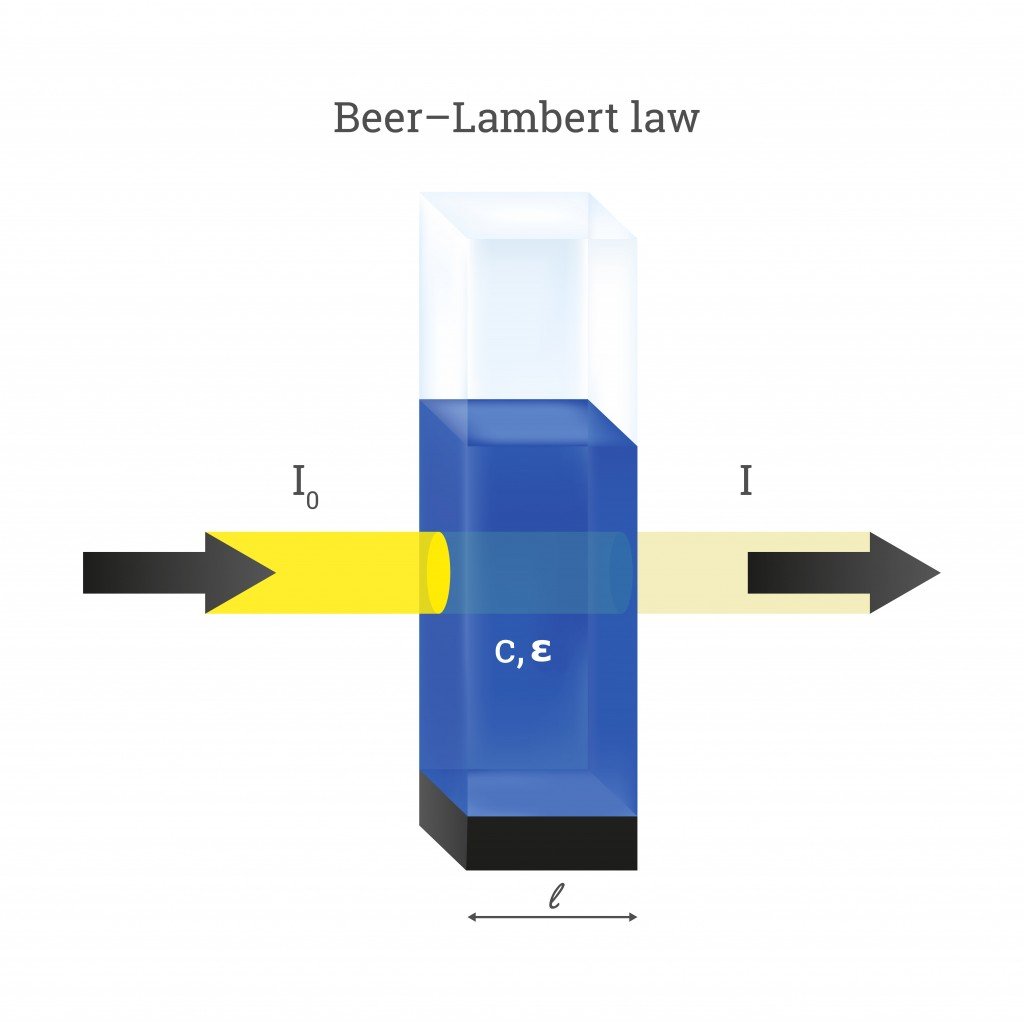
Beer's Law A=Kc . The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light. You can also use this calculator to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium.
from www.scienceabc.com
Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that a chemical solution's concentration is directly proportional to its light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. You can also use this calculator to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is.
Beers Law Definition, History, Equation, Formula And Example
Beer's Law A=Kc Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to determine the concentration of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download ID3102589 Beer's Law A=Kc Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that a chemical solution's concentration is directly proportional to its light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this. Beer's Law A=Kc.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID4449956 Beer's Law A=Kc Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light. The amount of light that a species absorbs in a spectroscopic transition can be. Beer's Law A=Kc.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer's Law A=Kc Beer's law states that a chemical solution's concentration is directly proportional to its light. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. You can also use this calculator to determine the concentration of. Since the concentration,. Beer's Law A=Kc.
From www.youtube.com
Ch 17 7 Beer's Law is Additive for All Components YouTube Beer's Law A=Kc Beer's law states that a chemical solution's concentration is directly proportional to its light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this. Beer's Law A=Kc.
From studylib.net
Beer`s Law Beer's Law A=Kc Beer's law states that a chemical solution's concentration is directly proportional to its light. You can also use this calculator to determine the concentration of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of. Beer's Law A=Kc.
From www.scribd.com
Beer S Law Plot of KMnO4 PDF PDF Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. You can also use this calculator to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by. Beer's Law A=Kc.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law A=Kc In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also. Beer's Law A=Kc.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light. Beer’s law, in spectroscopy, a relation concerning the absorption of. Beer's Law A=Kc.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law A=Kc You can also use this calculator to determine the concentration of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer's law states that a chemical solution's concentration is directly proportional to its light. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing. Beer's Law A=Kc.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer's law states that a chemical solution's concentration is directly proportional to its light. You can also use this calculator to determine the concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law A=Kc.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law A=Kc Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light. The amount of light that. Beer's Law A=Kc.
From www.slideserve.com
PPT Beers Law for a Single Component Sample PowerPoint Presentation, free download ID5860510 Beer's Law A=Kc In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Since the concentration, path length and molar absorptivity. Beer's Law A=Kc.
From www.youtube.com
BeerLambert law in easy way YouTube Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. You can also use this calculator to determine the concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that. Beer's Law A=Kc.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law A=Kc In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. You can also use this calculator to determine. Beer's Law A=Kc.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law A=Kc Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that a chemical solution's concentration is directly proportional to its light. The amount of light that. Beer's Law A=Kc.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry, Basic Introduction Beer's Law A=Kc In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number. Beer's Law A=Kc.
From www.pdfprof.com
Le Chatelierquestion Beer's Law A=Kc Beer's law states that a chemical solution's concentration is directly proportional to its light. You can also use this calculator to determine the concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The amount of light that a species absorbs in a. Beer's Law A=Kc.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. You can also use this calculator to determine the concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law A=Kc.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download ID2658679 Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known. Beer's Law A=Kc.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law A=Kc Beer's law states that a chemical solution's concentration is directly proportional to its light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b). Beer's Law A=Kc.
From www.chegg.com
Solved A Beer's Law plot is shown here along with the Beer's Law A=Kc You can also use this calculator to determine the concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer's law states that. Beer's Law A=Kc.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer's Law A=Kc Beer's law states that a chemical solution's concentration is directly proportional to its light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In most experiments, molar absorptivity (ε) and the length (b). Beer's Law A=Kc.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law PowerPoint Presentation ID5611981 Beer's Law A=Kc Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to determine the concentration of. Beer’s law, in spectroscopy, a relation concerning the. Beer's Law A=Kc.
From www.chegg.com
More Beer's Law Also known as the BeerLambert Law or Beer's Law A=Kc In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known. Beer's Law A=Kc.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download ID2658679 Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known. Beer's Law A=Kc.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer's Law A=Kc In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. You can also use this calculator to determine the concentration of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. Beer's Law A=Kc.
From www.slideserve.com
PPT chapter 2 PowerPoint Presentation, free download ID540228 Beer's Law A=Kc Beer's law states that a chemical solution's concentration is directly proportional to its light. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. You can also use this calculator to determine the concentration of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number. Beer's Law A=Kc.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Beer's Law A=Kc You can also use this calculator to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law states that a chemical solution's concentration is directly proportional to its light. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number. Beer's Law A=Kc.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 from Advanced Chemistry Beer's Law A=Kc Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to determine the concentration of. Beer's law states that a chemical solution's concentration is directly proportional to its light. The amount of light that a species absorbs in a. Beer's Law A=Kc.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer's Law A=Kc Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to determine the concentration of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs. Beer's Law A=Kc.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law A=Kc In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that a chemical solution's concentration is directly proportional to its light. Since the concentration, path length and molar absorptivity are all directly proportional to the. Beer's Law A=Kc.
From www.studocu.com
Chem 181 4 Beer's Law Fall 2020 Determining the Concentration of a Solution Beer’s Law Studocu Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light. You. Beer's Law A=Kc.
From www.slideserve.com
PPT Exp 14B Determining an Equilibrium Constant PowerPoint Presentation ID3200551 Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that a chemical solution's concentration is directly proportional to its light. In most experiments, molar absorptivity (ε) and the length (b). Beer's Law A=Kc.
From lukewarmtakes.net
Spectrophotometry and Beer's Law Lukewarm Takes Beer's Law A=Kc The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known. Beer's Law A=Kc.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law AP Chemistry Khan Beer's Law A=Kc Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. You can also use this calculator to determine the concentration of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law A=Kc.