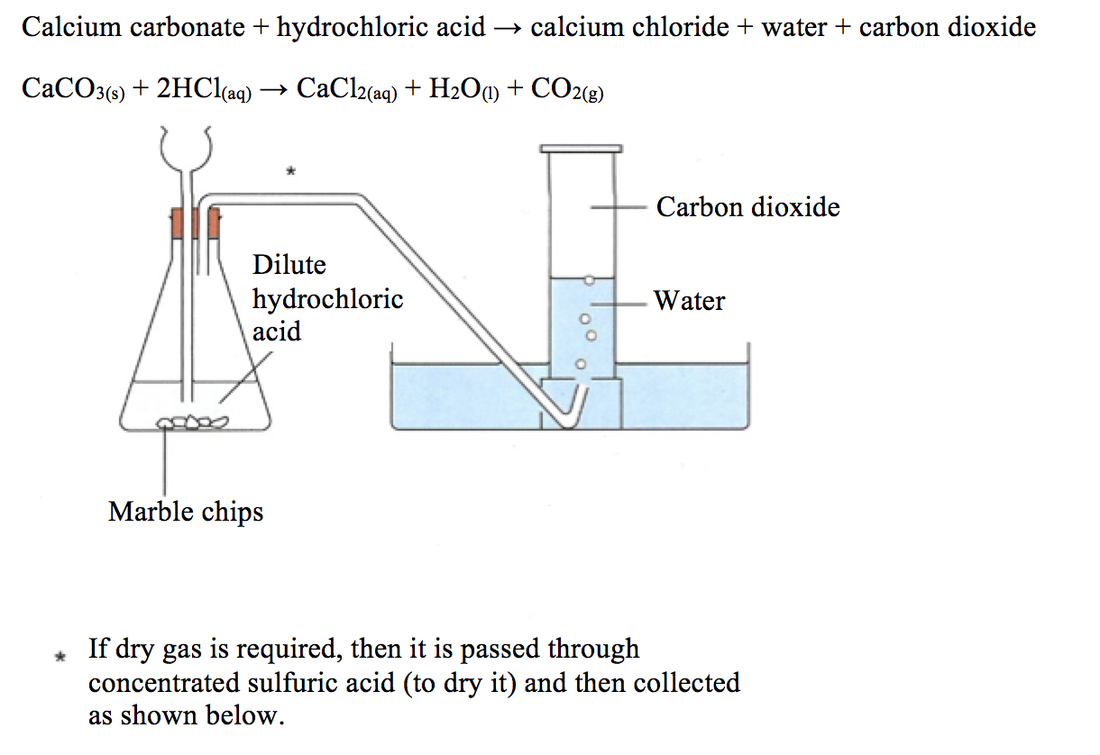
Lead Carbonate Equation For The Reaction . Reaction and net ionic equation. Chemical equation (h2o = h + o) 🛠️ Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Balance a chemical equation when given the. Enter an equation of an ionic chemical equation and press the balance button. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). If precipitation is expected, write a. Explain the roles of subscripts and coefficients in chemical equations. The balanced equation will be calculated along. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are.
from fyoawlxlr.blob.core.windows.net
The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Reaction and net ionic equation. Balance a chemical equation when given the. If precipitation is expected, write a. The balanced equation will be calculated along. Explain the roles of subscripts and coefficients in chemical equations. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds:
Lead Carbonate And Sulfuric Acid Equation at Bonnie Siemens blog
Lead Carbonate Equation For The Reaction Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. The balanced equation will be calculated along. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. If precipitation is expected, write a. Enter an equation of an ionic chemical equation and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Reaction and net ionic equation. Chemical equation (h2o = h + o) 🛠️ Balance a chemical equation when given the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions).
From www.numerade.com
SOLVED Write balanced complete chemical equation the complete ionic equation net ionic Lead Carbonate Equation For The Reaction Enter an equation of an ionic chemical equation and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Predict the result of mixing reasonably concentrated. Lead Carbonate Equation For The Reaction.
From www.fishersci.com
Lead(II) carbonate, ACS reagent, ACROS Organics Fisher Scientific Lead Carbonate Equation For The Reaction The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Balance a chemical equation when given the. If precipitation is expected, write a. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Reaction and net ionic equation. Explain. Lead Carbonate Equation For The Reaction.
From www.t3db.ca
T3DB Lead carbonate Lead Carbonate Equation For The Reaction Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Enter an equation of an ionic chemical equation and press the balance button. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Explain the roles of. Lead Carbonate Equation For The Reaction.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Lead Carbonate Equation For The Reaction Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. If precipitation is expected, write a. Reaction and net ionic equation. Balance a chemical equation when given the. Explain the roles of subscripts and coefficients in chemical equations. Chemical equation (h2o = h + o) 🛠️ Use the calculator below. Lead Carbonate Equation For The Reaction.
From fyoawlxlr.blob.core.windows.net
Lead Carbonate And Sulfuric Acid Equation at Bonnie Siemens blog Lead Carbonate Equation For The Reaction Balance a chemical equation when given the. The balanced equation will be calculated along. Reaction and net ionic equation. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Chemical equation (h2o = h + o) 🛠️ Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: If precipitation. Lead Carbonate Equation For The Reaction.
From www.numerade.com
Please help with this postlab!! Below are questions on my postlab! 1) 0.250g of calcium Lead Carbonate Equation For The Reaction Balance a chemical equation when given the. If precipitation is expected, write a. Explain the roles of subscripts and coefficients in chemical equations. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Enter an equation of an ionic chemical equation and press the balance button. Chemical equation (h2o = h + o) 🛠️ Write the dissolution equation. Lead Carbonate Equation For The Reaction.
From www.youtube.com
How to Write the Formula for Lead (II) bicarbonate YouTube Lead Carbonate Equation For The Reaction Explain the roles of subscripts and coefficients in chemical equations. Reaction and net ionic equation. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Balance a chemical equation when given the. If precipitation is expected, write a. The balanced equation will be calculated along. The chemical equation described in section 4.1. Lead Carbonate Equation For The Reaction.
From www.chegg.com
Solved 1. Shown above are the chemical equilibria of the Lead Carbonate Equation For The Reaction Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: The balanced equation will be calculated along. Enter an equation of an ionic chemical equation and press the balance button. Chemical equation (h2o = h + o) 🛠️ Use. Lead Carbonate Equation For The Reaction.
From www.numerade.com
Complete and balance the molecular equation for the reaction of aqueous sodium carbonate, Na2CO3 Lead Carbonate Equation For The Reaction Enter an equation of an ionic chemical equation and press the balance button. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The balanced equation will be calculated along. If precipitation is expected, write a. Balance a chemical equation when given. Lead Carbonate Equation For The Reaction.
From www.numerade.com
SOLVEDWrite a molecular equation for the precipitation reaction that occurs (if any) when each Lead Carbonate Equation For The Reaction Enter an equation of an ionic chemical equation and press the balance button. Chemical equation (h2o = h + o) 🛠️ The balanced equation will be calculated along. Reaction and net ionic equation. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide.. Lead Carbonate Equation For The Reaction.
From www.youtube.com
How to Balance PbCO3 + HCl = PbCl2 + CO2 + H2O YouTube Lead Carbonate Equation For The Reaction Chemical equation (h2o = h + o) 🛠️ Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Balance a chemical equation when given the. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. Use the calculator below to balance chemical equations and determine the type of. Lead Carbonate Equation For The Reaction.
From www.slideserve.com
PPT Writing Chemical Formulas PowerPoint Presentation, free download ID2434932 Lead Carbonate Equation For The Reaction The balanced equation will be calculated along. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Balance a chemical equation when given the. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Chemical equation (h2o = h + o) 🛠️ Enter an equation of an ionic chemical equation and press the. Lead Carbonate Equation For The Reaction.
From www.numerade.com
SOLVEDWhen solid lead(II) sulfide reacts with oxygen gas, the products are solid lead(II) oxide Lead Carbonate Equation For The Reaction Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Explain the roles of subscripts and coefficients in chemical equations. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Write the dissolution equation and the solubility product expression for. Lead Carbonate Equation For The Reaction.
From www.chegg.com
Solved Question 3 10 Points Lead (II) carbonate Lead Carbonate Equation For The Reaction The balanced equation will be calculated along. If precipitation is expected, write a. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction. Lead Carbonate Equation For The Reaction.
From brainly.in
write currently a balanced equation for the following word equation red lead gives you lead Lead Carbonate Equation For The Reaction Chemical equation (h2o = h + o) 🛠️ Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an equation of an ionic chemical equation and press the balance button. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If precipitation is expected, write a. The balanced equation will be. Lead Carbonate Equation For The Reaction.
From www.numerade.com
Lead (II) carbonate to form lead (II) oxide and carbon dioxide a) Write the balanced Lead Carbonate Equation For The Reaction If precipitation is expected, write a. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. The balanced equation will be calculated along. Chemical equation (h2o. Lead Carbonate Equation For The Reaction.
From www.geological-digressions.com
Mineralogy of carbonates; basic geochemistry Geological Digressions Lead Carbonate Equation For The Reaction Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. Reaction and net ionic equation. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). If precipitation is expected, write a. The balanced equation will. Lead Carbonate Equation For The Reaction.
From kunduz.com
[ANSWERED] Give the chemical formula for lead (IV) carbonate. Read Kunduz Lead Carbonate Equation For The Reaction Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an equation of an ionic chemical equation and press the balance button. Balance a chemical equation when given the. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Write the dissolution equation and the solubility product expression for each of. Lead Carbonate Equation For The Reaction.
From www.youtube.com
How to write chemical formula of Lead CarbonateLead carbonate formulaFormula of Lead carbonate Lead Carbonate Equation For The Reaction Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Explain the roles of subscripts and coefficients in chemical equations. Reaction and net ionic equation. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. Solid lead(ii) acetate is added to an. Lead Carbonate Equation For The Reaction.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid lead(II) sulfide reacts Lead Carbonate Equation For The Reaction Enter an equation of an ionic chemical equation and press the balance button. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Explain the roles of subscripts and coefficients in chemical. Lead Carbonate Equation For The Reaction.
From serc.carleton.edu
Figure 3 Carbonate Chemistry Lead Carbonate Equation For The Reaction Reaction and net ionic equation. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. The balanced equation will be calculated along. The chemical equation described in section 4.1 is. Lead Carbonate Equation For The Reaction.
From ocdrum.com
nickel(ii iodide and potassium carbonate balanced equation) Lead Carbonate Equation For The Reaction Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The balanced equation will be calculated along. If precipitation is expected, write a. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Enter an equation of an ionic chemical equation and press the. Lead Carbonate Equation For The Reaction.
From www.chegg.com
Solved Compare the solubility of lead carbonate in each of Lead Carbonate Equation For The Reaction Explain the roles of subscripts and coefficients in chemical equations. Reaction and net ionic equation. Enter an equation of an ionic chemical equation and press the balance button. If precipitation is expected, write a. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Use. Lead Carbonate Equation For The Reaction.
From studylib.net
File Lead Carbonate Equation For The Reaction The balanced equation will be calculated along. Reaction and net ionic equation. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Balance a chemical equation when given the. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Explain the roles of subscripts and coefficients in chemical equations. The. Lead Carbonate Equation For The Reaction.
From www.youtube.com
How to Write the Formula for Lead (IV) carbonate YouTube Lead Carbonate Equation For The Reaction Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Reaction and net ionic equation. Balance a chemical equation when given the. The balanced equation will be calculated along. The chemical equation described in section 4.1 is. Lead Carbonate Equation For The Reaction.
From www.youtube.com
How to write the equation for PbCO3 + H2O Lead (II) carbonate + Water YouTube Lead Carbonate Equation For The Reaction Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Chemical equation (h2o = h + o) 🛠️ The balanced equation will be calculated along. If precipitation is expected, write a. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Balance a chemical equation when given the. Enter an equation of. Lead Carbonate Equation For The Reaction.
From gioobnpqg.blob.core.windows.net
Lead Carbonate Balanced Equation at Lyle Hill blog Lead Carbonate Equation For The Reaction Reaction and net ionic equation. The balanced equation will be calculated along. Balance a chemical equation when given the. Enter an equation of an ionic chemical equation and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Chemical equation (h2o = h. Lead Carbonate Equation For The Reaction.
From www.numerade.com
SOLVED Part Write a balanced equation for the of lead (Il) carbonate to yield Lead Carbonate Equation For The Reaction Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Balance a chemical equation when given the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Enter an equation of an ionic chemical. Lead Carbonate Equation For The Reaction.
From www.numerade.com
SOLVED The preparation of lead sulphate from lead carbonate is a two step process. (Lead Lead Carbonate Equation For The Reaction Explain the roles of subscripts and coefficients in chemical equations. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). The balanced equation will be calculated along. Chemical equation (h2o = h + o) 🛠️ The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in. Lead Carbonate Equation For The Reaction.
From www.numerade.com
SOLVEDWrite a molecular equation for the precipitation reaction that occurs (if any) when the Lead Carbonate Equation For The Reaction Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Chemical equation (h2o = h + o) 🛠️ Enter an equation of an ionic chemical equation and press the balance button. If precipitation is expected, write a. Write the dissolution equation and. Lead Carbonate Equation For The Reaction.
From www.chegg.com
Solved Question 3 10 Points Lead (II) carbonate Lead Carbonate Equation For The Reaction Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Explain the roles of subscripts and coefficients in chemical equations. Enter an equation of an ionic chemical equation and press the balance button. Write the dissolution equation and the solubility product expression for each of. Lead Carbonate Equation For The Reaction.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Lead Carbonate Equation For The Reaction Explain the roles of subscripts and coefficients in chemical equations. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: If precipitation is expected, write a. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. Enter. Lead Carbonate Equation For The Reaction.
From www.slideserve.com
PPT 9E Reactions of metals and their compounds PowerPoint Presentation ID2132665 Lead Carbonate Equation For The Reaction The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: The balanced equation will be calculated along. Enter an equation of an ionic chemical equation and press the balance. Lead Carbonate Equation For The Reaction.
From www.toppr.com
Identify the type of reactions taking place in each of the following cases and write the Lead Carbonate Equation For The Reaction The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Reaction and net ionic equation. Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. The balanced equation will be. Lead Carbonate Equation For The Reaction.
From www.youtube.com
Reactions between Metal Carbonates and Acids YouTube Lead Carbonate Equation For The Reaction Chemical equation (h2o = h + o) 🛠️ The balanced equation will be calculated along. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds: Solid lead(ii) acetate is added to an aqueous solution of ammonium iodide. The chemical. Lead Carbonate Equation For The Reaction.