Redox Titration End Point . This method relies on the. The oxidized and reduced forms of some titrants, such as mno 4. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. An example of redox titration is the treatment of an iodine solution. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. Three types of indicators are used to signal a redox titration’s end point. It may involve the use of a redox indicator and/or a potentiometer. A common example of a redox titration is the treatment of a solution of iodine. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while.
from www.chegg.com
Three types of indicators are used to signal a redox titration’s end point. This method relies on the. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while. A common example of a redox titration is the treatment of a solution of iodine. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. It may involve the use of a redox indicator and/or a potentiometer. An example of redox titration is the treatment of an iodine solution. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The oxidized and reduced forms of some titrants, such as mno 4.
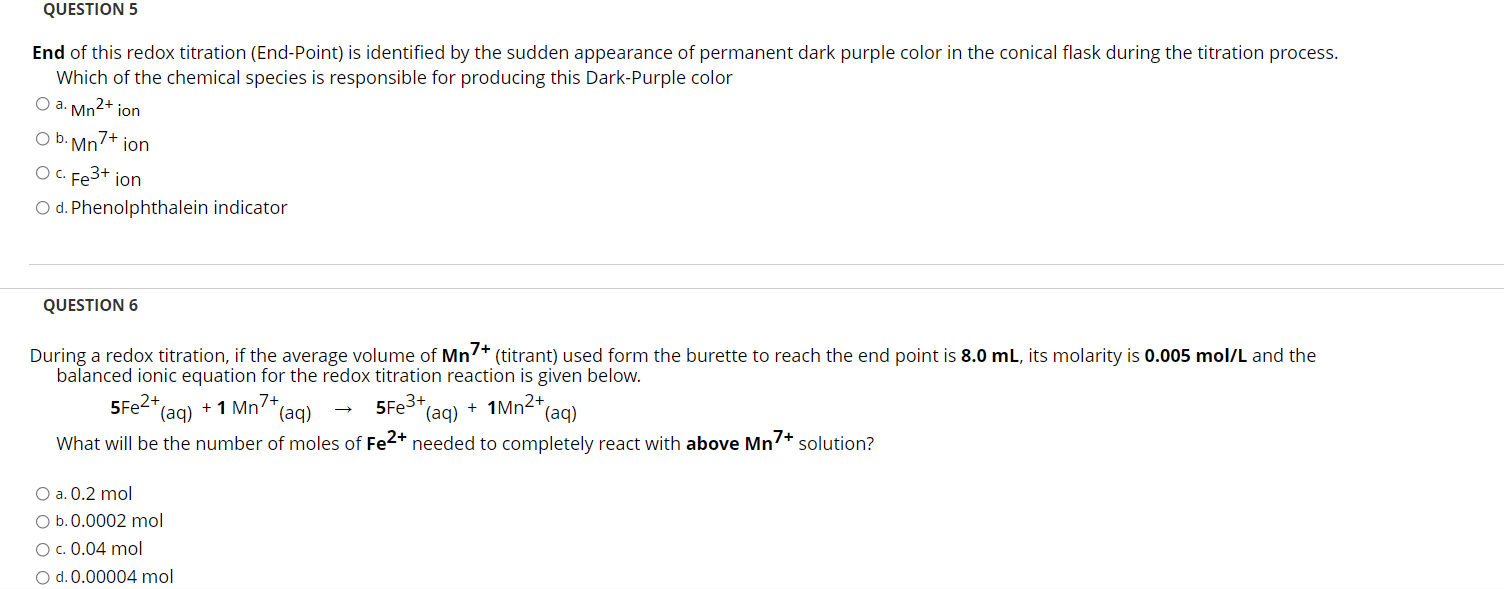
Solved QUESTION 5 End of this redox titration (EndPoint) is
Redox Titration End Point This method relies on the. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while. Three types of indicators are used to signal a redox titration’s end point. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. An example of redox titration is the treatment of an iodine solution. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The oxidized and reduced forms of some titrants, such as mno 4. It may involve the use of a redox indicator and/or a potentiometer. This method relies on the. A common example of a redox titration is the treatment of a solution of iodine.
From www.chegg.com
Solved Lab 5 A Redox Titration and the Oxidizing Power of Redox Titration End Point Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. This method relies on the. It may involve the use of a redox indicator and/or a potentiometer. Thus, it can be understood that redox titrations involve a transfer of electrons between the. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration End Point Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. It may involve the use of a redox indicator and/or a potentiometer. An example of redox titration is the treatment of an iodine solution. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Redox Titration End Point A common example of a redox titration is the treatment of a solution of iodine. This method relies on the. It may involve the use of a redox indicator and/or a potentiometer. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound.. Redox Titration End Point.
From www.studyread.com
Redox Titrations The Experimental steps and Applications Redox Titration End Point Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. A common example of a redox titration is the treatment of a solution of iodine. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element. Redox Titration End Point.
From chemistnotes.com
Redox Titration Definition, Requirements, Indicators, and 5 Reliable Applications Chemistry Notes Redox Titration End Point Three types of indicators are used to signal a redox titration’s end point. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. It may involve the use of a. Redox Titration End Point.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Redox Titration End Point By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. Three types of indicators are used to signal a redox titration’s end point. Use our revision notes to understand how. Redox Titration End Point.
From www.lsua.us
No Slide Title Redox Titration End Point This method relies on the. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while. It may involve the use of a redox indicator and/or a potentiometer. An example of redox titration is the treatment of an iodine solution. The oxidized and reduced forms of some titrants, such. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration End Point Three types of indicators are used to signal a redox titration’s end point. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. Use our revision notes to understand how. Redox Titration End Point.
From www.brainkart.com
Selecting and Evaluating the End Point Titrations Based on Redox Reactions Redox Titration End Point This method relies on the. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. Another method for locating a redox titration’s end point is a potentiometric titration in which. Redox Titration End Point.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Redox Titration End Point Three types of indicators are used to signal a redox titration’s end point. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a. Redox Titration End Point.
From www.youtube.com
Redox Titration End Point YouTube Redox Titration End Point This method relies on the. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Three types of indicators are used to signal a redox titration’s end point. It may involve the use of a redox indicator and/or a potentiometer. A common. Redox Titration End Point.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Redox Titration End Point An example of redox titration is the treatment of an iodine solution. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in. Redox Titration End Point.
From slidetodoc.com
Chapter 15 Redox Titrations 15 1 The Shape Redox Titration End Point Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. An example of redox titration is the treatment of an iodine solution. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in. Redox Titration End Point.
From askfilo.com
point, End point, Titration error2. What is redox titration? Give an exa.. Redox Titration End Point Three types of indicators are used to signal a redox titration’s end point. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration End Point Three types of indicators are used to signal a redox titration’s end point. This method relies on the. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. A common example of a redox titration is the treatment of a solution of. Redox Titration End Point.
From www.chegg.com
Solved QUESTION 5 End of this redox titration (EndPoint) is Redox Titration End Point A common example of a redox titration is the treatment of a solution of iodine. The oxidized and reduced forms of some titrants, such as mno 4. An example of redox titration is the treatment of an iodine solution. This method relies on the. Another method for locating a redox titration’s end point is a potentiometric titration in which we. Redox Titration End Point.
From www.slideserve.com
PPT Redox Stoichiometry PowerPoint Presentation, free download ID2489846 Redox Titration End Point Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. A common example of a redox titration is the treatment. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration End Point An example of redox titration is the treatment of an iodine solution. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant.. Redox Titration End Point.
From studylib.net
Redox Titrations Introduction 1.) Redox Titration Redox Titration End Point This method relies on the. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. Another method for locating a redox titration’s. Redox Titration End Point.
From scienceinfo.com
Redox titration Types, Applications, Advantages, Disadvantages Redox Titration End Point It may involve the use of a redox indicator and/or a potentiometer. This method relies on the. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. The oxidized and. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Redox Titration End Point The oxidized and reduced forms of some titrants, such as mno 4. An example of redox titration is the treatment of an iodine solution. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. This method relies on the. Another method for locating a redox titration’s end point is a. Redox Titration End Point.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Redox Titration End Point The oxidized and reduced forms of some titrants, such as mno 4. It may involve the use of a redox indicator and/or a potentiometer. An example of redox titration is the treatment of an iodine solution. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. This method relies on. Redox Titration End Point.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Redox Titration End Point This method relies on the. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while. An example of redox titration is the treatment of an iodine solution. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage. Redox Titration End Point.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Redox Titration End Point It may involve the use of a redox indicator and/or a potentiometer. An example of redox titration is the treatment of an iodine solution. A common example of a redox titration is the treatment of a solution of iodine. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Redox Titration End Point This method relies on the. Three types of indicators are used to signal a redox titration’s end point. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. Use our. Redox Titration End Point.
From www.vrogue.co
Titration 4 Redox Titration End Point Titration Scree vrogue.co Redox Titration End Point Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Three types of indicators are used to signal a redox titration’s end point. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Redox Titration End Point Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while. Use our revision notes to understand how a redox titration in a level chemistry can be used to. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Redox Titration End Point By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the. Redox Titration End Point.
From www.studypug.com
Master Redox Titrations Essential Analytical Chemistry Skill StudyPug Redox Titration End Point Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while. An example of redox titration is the treatment of an iodine solution. The oxidized and reduced forms of some titrants, such as mno 4. It may involve the use of a redox indicator and/or a potentiometer. Thus, it. Redox Titration End Point.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download ID3221319 Redox Titration End Point Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. Three types of indicators are used to signal a redox titration’s end point. An example of redox titration is the treatment of an iodine solution. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the. Redox Titration End Point.
From www.chegg.com
Solved The redox titration reached the endpoint when 0.01924 Redox Titration End Point An example of redox titration is the treatment of an iodine solution. The oxidized and reduced forms of some titrants, such as mno 4. Three types of indicators are used to signal a redox titration’s end point. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. Another method for. Redox Titration End Point.
From slidetodoc.com
Redox Titrations Introduction 1 Redox Titration Based on Redox Titration End Point Three types of indicators are used to signal a redox titration’s end point. It may involve the use of a redox indicator and/or a potentiometer. Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant. A common example of a redox titration is the treatment of a solution of iodine.. Redox Titration End Point.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID3080571 Redox Titration End Point Three types of indicators are used to signal a redox titration’s end point. By employing a suitable indicator electrode in conjunction with a potentiometric setup and measuring the potential that develops in the reaction mixture as a function of the added volume of titrant, the endpoint of a redox titration can be identified. Another method for locating a redox titration’s. Redox Titration End Point.
From www.slideserve.com
PPT 3 rd lecture PowerPoint Presentation, free download ID2145155 Redox Titration End Point Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while. An example of redox titration is the treatment of an iodine solution. The oxidized and reduced forms of some titrants, such as mno 4. It may involve the use of a redox indicator and/or a potentiometer. Three types. Redox Titration End Point.
From www.brainkart.com
Selecting and Evaluating the End Point Titrations Based on Redox Reactions Redox Titration End Point Three types of indicators are used to signal a redox titration’s end point. Another method for locating a redox titration’s end point is a potentiometric titration in which we monitor the change in potential while. This method relies on the. An example of redox titration is the treatment of an iodine solution. It may involve the use of a redox. Redox Titration End Point.