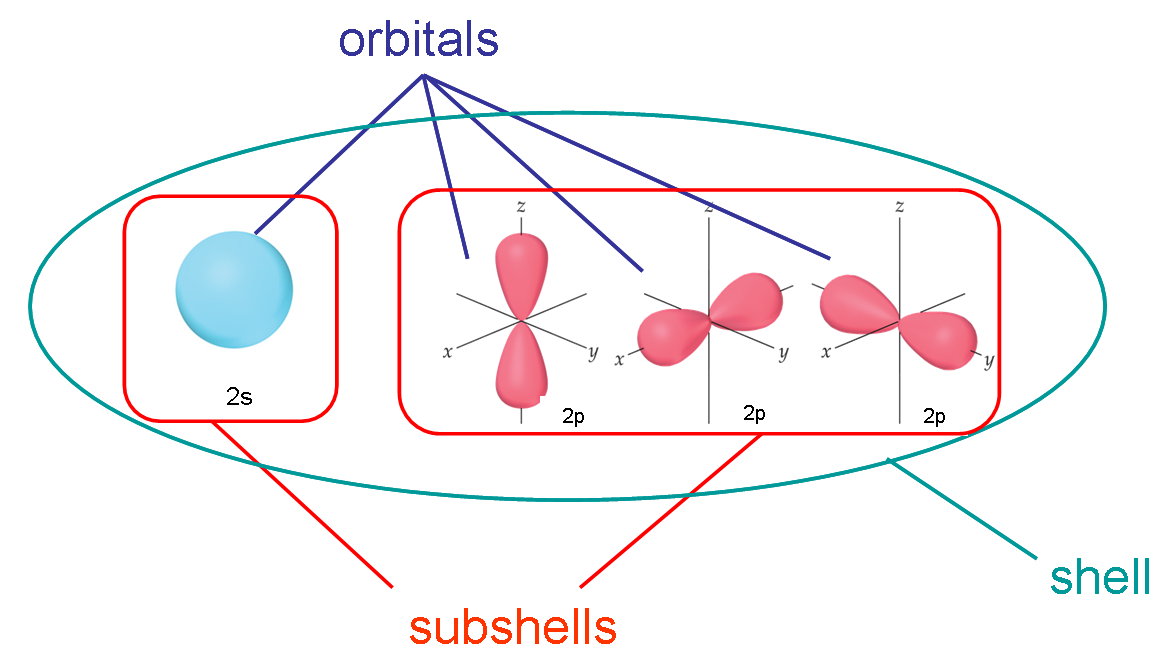
Shell Definition Electrons . As we discussed previously, this does. Each allowed electron orbit is. The shells are orbital paths that are followed by electrons around the nucleus. The innermost shell has a. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. Learn how many electrons are found in each shell with the rule, order, and. The energy levels for the electrons in an atom are often referred to as electron shells. What is an electron shell with a model and diagram.
from socratic.org
The energy levels for the electrons in an atom are often referred to as electron shells. Each allowed electron orbit is. The innermost shell has a. What is an electron shell with a model and diagram. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The shells are orbital paths that are followed by electrons around the nucleus. Learn how many electrons are found in each shell with the rule, order, and. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus.
What is the difference between electron shells and electron orbitals? Socratic
Shell Definition Electrons An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. The energy levels for the electrons in an atom are often referred to as electron shells. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Learn how many electrons are found in each shell with the rule, order, and. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The shells are orbital paths that are followed by electrons around the nucleus. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. The innermost shell has a. As we discussed previously, this does. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. Each allowed electron orbit is. What is an electron shell with a model and diagram.
From ar.inspiredpencil.com
Periodic Table Electron Shells Shell Definition Electrons The shells are orbital paths that are followed by electrons around the nucleus. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. An electron shell is a collection of orbitals that share the same principal quantum number,. Shell Definition Electrons.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science Department. Shell Definition Electrons The shells are orbital paths that are followed by electrons around the nucleus. The energy levels for the electrons in an atom are often referred to as electron shells. As we discussed previously, this does. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. In very simple terms. Shell Definition Electrons.
From socratic.org
What is the difference between electron shells and electron orbitals? Socratic Shell Definition Electrons An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. The energy levels for the electrons in an atom are often referred to as electron shells. What is an electron shell with a model and diagram. The innermost shell has a. Each allowed electron orbit is. Learn how many electrons. Shell Definition Electrons.
From byjus.com
Different energy shells, valence shell, valence electrons Shell Definition Electrons What is an electron shell with a model and diagram. The innermost shell has a. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Learn how many electrons are found in each shell with the rule, order, and. Electron shell, regions surrounding the atomic nucleus containing a specific number. Shell Definition Electrons.
From www.nagwa.com
Lesson Video Electron Shells Nagwa Shell Definition Electrons The energy levels for the electrons in an atom are often referred to as electron shells. The shells are orbital paths that are followed by electrons around the nucleus. As we discussed previously, this does. Learn how many electrons are found in each shell with the rule, order, and. Electron shell, regions surrounding the atomic nucleus containing a specific number. Shell Definition Electrons.
From slidetodoc.com
Valence Electrons ELECTRONS AVAILABLE FOR BONDING NC Standards Shell Definition Electrons Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. What is an electron shell with a model and diagram. Each allowed electron orbit is. In very simple terms. Shell Definition Electrons.
From www.youtube.com
Electron shells Elements 118 YouTube Shell Definition Electrons Each allowed electron orbit is. Learn how many electrons are found in each shell with the rule, order, and. What is an electron shell with a model and diagram. The shells are orbital paths that are followed by electrons around the nucleus. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic. Shell Definition Electrons.
From bmp-level.blogspot.com
What Is An Electron Shell Definition bmplevel Shell Definition Electrons As we discussed previously, this does. The energy levels for the electrons in an atom are often referred to as electron shells. The shells are orbital paths that are followed by electrons around the nucleus. The innermost shell has a. Each allowed electron orbit is. What is an electron shell with a model and diagram. An electron shell is a. Shell Definition Electrons.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Shell Definition Electrons Each allowed electron orbit is. The shells are orbital paths that are followed by electrons around the nucleus. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. What is an electron shell with a model and diagram. The innermost shell has a. In very simple terms an electron shell is the outside part of an atom. Shell Definition Electrons.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Shell Definition Electrons Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. Learn how many electrons are found in each shell with the rule, order, and. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. What is an electron shell with a model and diagram. In very. Shell Definition Electrons.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Shell Definition Electrons The shells are orbital paths that are followed by electrons around the nucleus. Learn how many electrons are found in each shell with the rule, order, and. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. As we discussed previously, this does. The innermost shell has a. An electron shell is a collection of orbitals that. Shell Definition Electrons.
From www.slideserve.com
PPT BIOLOGY EOC REVIEW PowerPoint Presentation, free download ID3432179 Shell Definition Electrons Each allowed electron orbit is. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The shells are orbital paths that are followed by electrons around the nucleus. The. Shell Definition Electrons.
From mungfali.com
How Many Electrons Are In Each Shell Shell Definition Electrons Each allowed electron orbit is. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. What is an electron shell with a model and diagram. Under standard conditions, atoms fill the inner shells first, often resulting in a. Shell Definition Electrons.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Shell Definition Electrons Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. The innermost shell has a. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. Learn how many. Shell Definition Electrons.
From ar.inspiredpencil.com
Electron Orbital Shells Shell Definition Electrons Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The shells are orbital paths that are followed by electrons around the nucleus. The innermost shell has a. Each allowed electron orbit is. An electron shell is a collection of orbitals that share the same principal quantum number, representing. Shell Definition Electrons.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science Shell Definition Electrons As we discussed previously, this does. Learn how many electrons are found in each shell with the rule, order, and. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The innermost shell has a. What is an. Shell Definition Electrons.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica Shell Definition Electrons Each allowed electron orbit is. The innermost shell has a. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Learn how many electrons are found in each shell with the rule, order, and. In very. Shell Definition Electrons.
From www.animalia-life.club
Electron Shell Diagram Shell Definition Electrons Each allowed electron orbit is. What is an electron shell with a model and diagram. The energy levels for the electrons in an atom are often referred to as electron shells. The innermost shell has a. Learn how many electrons are found in each shell with the rule, order, and. Electron shell, regions surrounding the atomic nucleus containing a specific. Shell Definition Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Shell Definition Electrons Learn how many electrons are found in each shell with the rule, order, and. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. The energy levels for the electrons in an atom are often referred to as electron shells. Each allowed electron orbit is. What is an electron shell with. Shell Definition Electrons.
From ar.inspiredpencil.com
Electron Orbital Shells Shell Definition Electrons An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. The shells are orbital paths that are followed by electrons around the nucleus. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The energy levels for the electrons in an atom are often referred to as. Shell Definition Electrons.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary Shell Definition Electrons As we discussed previously, this does. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. What is an electron shell with a model and diagram. The innermost shell has a. Each allowed electron orbit is. Learn how many electrons are found in each shell with the rule, order, and. The. Shell Definition Electrons.
From surfguppy.com
Valence Electrons Definition, Obits and Energy Level Shell Definition Electrons As we discussed previously, this does. The shells are orbital paths that are followed by electrons around the nucleus. The energy levels for the electrons in an atom are often referred to as electron shells. What is an electron shell with a model and diagram. Each allowed electron orbit is. Electron shell, regions surrounding the atomic nucleus containing a specific. Shell Definition Electrons.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry Shell Definition Electrons Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. As we discussed previously, this does. Each allowed electron orbit is. The energy levels for the electrons in an atom are often referred to as electron shells. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost. Shell Definition Electrons.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Shell Definition Electrons Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. What is an electron shell with a model and diagram. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. In very simple terms an electron shell is the outside part of an atom that surrounds. Shell Definition Electrons.
From www.youtube.com
mr i explains Electron Shells (GCSE Chemistry) YouTube Shell Definition Electrons An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Each allowed electron orbit is. The energy levels for the electrons in an atom are often referred to as electron shells. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the. Shell Definition Electrons.
From www.slideserve.com
PPT Atoms and Ions PowerPoint Presentation, free download ID2050882 Shell Definition Electrons An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Learn how many electrons are found in each shell with the rule, order, and. The innermost shell has a. Each allowed electron orbit is. The shells are orbital paths that are followed by electrons around the nucleus. As we discussed. Shell Definition Electrons.
From www.britannica.com
Electron shell Definition & Facts Britannica Shell Definition Electrons What is an electron shell with a model and diagram. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The energy levels for the electrons in an atom are often referred to as electron shells. The innermost shell has a. The shells are orbital paths that are followed by electrons around the nucleus. Under standard conditions,. Shell Definition Electrons.
From keystagewiki.com
Electron Orbital Key Stage Wiki Shell Definition Electrons The energy levels for the electrons in an atom are often referred to as electron shells. As we discussed previously, this does. What is an electron shell with a model and diagram. The shells are orbital paths that are followed by electrons around the nucleus. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number. Shell Definition Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Shell Definition Electrons An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. The energy levels for the electrons in an atom are often referred to as electron shells. Each allowed electron orbit is.. Shell Definition Electrons.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 Shell Definition Electrons Learn how many electrons are found in each shell with the rule, order, and. Each allowed electron orbit is. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell.. Shell Definition Electrons.
From study.com
Electron Shell Definition, Energy Levels & Valence Video & Lesson Transcript Shell Definition Electrons An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Each allowed electron orbit is. The shells are orbital paths that are followed by electrons around the nucleus. What is an electron shell with a model and diagram. Learn how many electrons are found in each shell with the rule,. Shell Definition Electrons.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Shell Definition Electrons Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. As we discussed previously, this does. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Each allowed electron orbit is. The shells are orbital paths that are followed by electrons around the nucleus. The energy levels. Shell Definition Electrons.
From pediaa.com
Difference Between Shell Subshell and Orbital Definition, Structure, Properties Shell Definition Electrons Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. Learn how many electrons are found in each shell with the rule, order, and. In very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. What is an electron shell with. Shell Definition Electrons.
From mungfali.com
What Is An Electron Shell Shell Definition Electrons Each allowed electron orbit is. Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The. Shell Definition Electrons.
From lessonfullgalleting.z21.web.core.windows.net
Structure Of Atom Bohr Model Shell Definition Electrons The energy levels for the electrons in an atom are often referred to as electron shells. The innermost shell has a. An electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. Each allowed electron orbit is. What is an electron shell with a model and diagram. The shells are orbital. Shell Definition Electrons.