Iron Electron Configuration Valence Electrons . Iron can also give up either of the paired electrons from a 3d orbital. Iron is a transition metal, so the number of valence electrons includes those in the 3d. But for most of the transition and inner. As a result, iron has eight valence electrons. As previously stated, iron has two valence states: The total number of valence electrons for iron is 8: As a result, when it gives up the two 4s electrons, it gains a valency of +2. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as.
from www.sciencefacts.net
Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. But for most of the transition and inner. Iron is a transition metal, so the number of valence electrons includes those in the 3d. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. As previously stated, iron has two valence states: The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. As a result, when it gives up the two 4s electrons, it gains a valency of +2. As a result, iron has eight valence electrons. Iron can also give up either of the paired electrons from a 3d orbital. The total number of valence electrons for iron is 8:
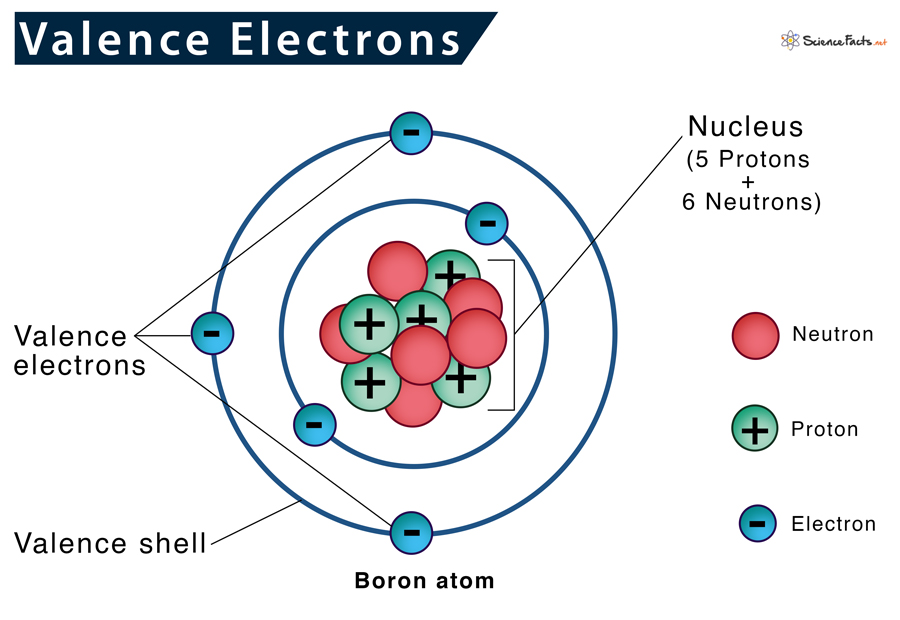
Valence Electrons Definition, Location, Importance, and Diagram
Iron Electron Configuration Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. As previously stated, iron has two valence states: Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. As a result, when it gives up the two 4s electrons, it gains a valency of +2. But for most of the transition and inner. Iron can also give up either of the paired electrons from a 3d orbital. Iron is a transition metal, so the number of valence electrons includes those in the 3d. The total number of valence electrons for iron is 8: As a result, iron has eight valence electrons.
From www.britannica.com
Iron Element, Occurrence, Uses, Properties, & Compounds Britannica Iron Electron Configuration Valence Electrons As previously stated, iron has two valence states: As a result, when it gives up the two 4s electrons, it gains a valency of +2. As a result, iron has eight valence electrons. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed. Iron Electron Configuration Valence Electrons.
From www.youtube.com
Valence Electrons for Fe (Iron) YouTube Iron Electron Configuration Valence Electrons As a result, iron has eight valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. As previously stated, iron has two valence states: The total number of valence electrons for iron is 8: But for most of the transition and. Iron Electron Configuration Valence Electrons.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Iron Electron Configuration Valence Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Iron can also give up. Iron Electron Configuration Valence Electrons.
From www.expii.com
Valence Electrons — Definition & Importance Expii Iron Electron Configuration Valence Electrons As a result, iron has eight valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and. Iron Electron Configuration Valence Electrons.
From valenceelectrons.com
How many valence electrons does silicon(Si) have? Iron Electron Configuration Valence Electrons Iron can also give up either of the paired electrons from a 3d orbital. Iron is a transition metal, so the number of valence electrons includes those in the 3d. As a result, when it gives up the two 4s electrons, it gains a valency of +2. As previously stated, iron has two valence states: How to write the electron. Iron Electron Configuration Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Iron Electron Configuration Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. As previously stated, iron has two valence states: Iron can also give up either of the paired electrons from a 3d orbital. As a result, iron has eight valence electrons. How to write. Iron Electron Configuration Valence Electrons.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Iron Electron Configuration Valence Electrons As a result, iron has eight valence electrons. As a result, when it gives up the two 4s electrons, it gains a valency of +2. Iron can also give up either of the paired electrons from a 3d orbital. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to. Iron Electron Configuration Valence Electrons.
From periodictable.me
How Many Valence Electrons Does Iron have Archives Dynamic Periodic Iron Electron Configuration Valence Electrons As a result, when it gives up the two 4s electrons, it gains a valency of +2. Iron is a transition metal, so the number of valence electrons includes those in the 3d. The total number of valence electrons for iron is 8: Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26. Iron Electron Configuration Valence Electrons.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Electron Configuration Valence Electrons But for most of the transition and inner. The total number of valence electrons for iron is 8: Iron can also give up either of the paired electrons from a 3d orbital. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. As. Iron Electron Configuration Valence Electrons.
From www.numerade.com
SOLVED18. Write out the electron configuration for Fe" , Fe'? and Fe Iron Electron Configuration Valence Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. The total number of. Iron Electron Configuration Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Electron Configuration Valence Electrons As previously stated, iron has two valence states: Iron can also give up either of the paired electrons from a 3d orbital. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. The total number of valence electrons for iron is 8: As. Iron Electron Configuration Valence Electrons.
From new--electronic.blogspot.com
Electron Configuration Of Ferrous Ion Iron Electron Configuration Valence Electrons Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. As a result, when it gives up the two 4s electrons, it gains a valency of +2. How to write the electron configuration for iron (fe) in order to write the iron. Iron Electron Configuration Valence Electrons.
From quizlet.com
Draw the electron configuration for a neutral atom of iron. Quizlet Iron Electron Configuration Valence Electrons Iron is a transition metal, so the number of valence electrons includes those in the 3d. But for most of the transition and inner. As a result, iron has eight valence electrons. Iron can also give up either of the paired electrons from a 3d orbital. As a result, when it gives up the two 4s electrons, it gains a. Iron Electron Configuration Valence Electrons.
From ceguvkcr.blob.core.windows.net
What Kind Of Orbital Does Iron's Electron Configuration End With at Iron Electron Configuration Valence Electrons As a result, iron has eight valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. As previously stated, iron has two valence states: Iron is a transition metal, so the number of valence electrons includes those in the 3d. But. Iron Electron Configuration Valence Electrons.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Iron Electron Configuration Valence Electrons Iron is a transition metal, so the number of valence electrons includes those in the 3d. But for most of the transition and inner. As a result, when it gives up the two 4s electrons, it gains a valency of +2. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s. Iron Electron Configuration Valence Electrons.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Iron Electron Configuration Valence Electrons Iron can also give up either of the paired electrons from a 3d orbital. As a result, when it gives up the two 4s electrons, it gains a valency of +2. As a result, iron has eight valence electrons. Iron is a transition metal, so the number of valence electrons includes those in the 3d. The electron configuration of an. Iron Electron Configuration Valence Electrons.
From valenceelectrons.com
Iron(Fe) electron configuration and orbital diagram Iron Electron Configuration Valence Electrons The total number of valence electrons for iron is 8: Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. As a result, iron has eight valence electrons. As a result, when it gives up the two 4s electrons, it gains a. Iron Electron Configuration Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Electron Configuration Valence Electrons But for most of the transition and inner. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. As a result, iron has eight valence electrons. The total number of valence electrons for iron is 8: Iron can also give up either of. Iron Electron Configuration Valence Electrons.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration Iron Electron Configuration Valence Electrons The total number of valence electrons for iron is 8: As previously stated, iron has two valence states: Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. How to write the electron configuration for iron (fe) in order to write the. Iron Electron Configuration Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Electron Configuration Valence Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. Iron can also give up either of the paired electrons from a 3d orbital. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with. Iron Electron Configuration Valence Electrons.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Electron Configuration Valence Electrons As previously stated, iron has two valence states: The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. The total number of valence electrons for iron is 8: But for most of the transition and inner. Iron can also give up either of. Iron Electron Configuration Valence Electrons.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Electron Configuration Valence Electrons Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. As previously stated, iron. Iron Electron Configuration Valence Electrons.
From www.youtube.com
Valence Electrons & Electron Configurations YouTube Iron Electron Configuration Valence Electrons The total number of valence electrons for iron is 8: The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. Iron Electron Configuration Valence Electrons.
From utedzz.blogspot.com
Periodic Table Iron Valence Electrons Periodic Table Timeline Iron Electron Configuration Valence Electrons Iron can also give up either of the paired electrons from a 3d orbital. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. Iron is a transition metal, so the number of valence electrons includes those in the 3d. Iron is. Iron Electron Configuration Valence Electrons.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Iron Electron Configuration Valence Electrons As a result, when it gives up the two 4s electrons, it gains a valency of +2. As a result, iron has eight valence electrons. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. The electron configuration of an iron atom. Iron Electron Configuration Valence Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Iron Electron Configuration Valence Electrons As a result, iron has eight valence electrons. As a result, when it gives up the two 4s electrons, it gains a valency of +2. The total number of valence electrons for iron is 8: How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of. Iron Electron Configuration Valence Electrons.
From awesomehome.co
Iron Periodic Table Electrons Awesome Home Iron Electron Configuration Valence Electrons Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. As previously stated, iron has two valence states: Iron can also give up either of the paired electrons from a 3d orbital. Iron is a transition metal, so the number of valence. Iron Electron Configuration Valence Electrons.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Iron Electron Configuration Valence Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. But for most of the. Iron Electron Configuration Valence Electrons.
From edwardhall.z19.web.core.windows.net
Valence Electrons Periodic Table Chart Iron Electron Configuration Valence Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. As previously stated, iron. Iron Electron Configuration Valence Electrons.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Electron Configuration Valence Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and is classed as. Iron is a transition. Iron Electron Configuration Valence Electrons.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic Iron Electron Configuration Valence Electrons How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. As a result, when it gives up the two 4s electrons, it gains a valency of +2. But for most of the transition and inner. Iron can also give up either of. Iron Electron Configuration Valence Electrons.
From www.alamy.com
Iron Chemical Element Stock Photos & Iron Chemical Element Stock Images Iron Electron Configuration Valence Electrons Iron is a transition metal, so the number of valence electrons includes those in the 3d. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2. Iron Electron Configuration Valence Electrons.
From sciencenotes.org
List of Electron Configurations of Elements Iron Electron Configuration Valence Electrons Iron can also give up either of the paired electrons from a 3d orbital. How to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the number of electrons for the. But for most of the transition and inner. As a result, when it gives up the two 4s. Iron Electron Configuration Valence Electrons.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Iron Electron Configuration Valence Electrons The total number of valence electrons for iron is 8: But for most of the transition and inner. As a result, when it gives up the two 4s electrons, it gains a valency of +2. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2. Iron Electron Configuration Valence Electrons.
From www.toppr.com
Electronic Configuration of Iron Fe element Valency, Applications Iron Electron Configuration Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. But for most of the transition and inner. Iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of 55.8452 u and. Iron Electron Configuration Valence Electrons.