Salt Vs Baking Soda Electrolysis . Electrolytes) is great to use in the electrolysis of water. Some examples of electrolytes you. Separating hydrogen and oxygen from water is done through electrolysis. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. To do this experiment, you will need the following items: In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. Table salt (kosher salt is best since it’s. What electrolyte should i use in the water? Mainly anything that contains ions (i.e.
from ptx-hub.org
Table salt (kosher salt is best since it’s. Some examples of electrolytes you. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Mainly anything that contains ions (i.e. What electrolyte should i use in the water? To do this experiment, you will need the following items: Separating hydrogen and oxygen from water is done through electrolysis. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Electrolytes) is great to use in the electrolysis of water.
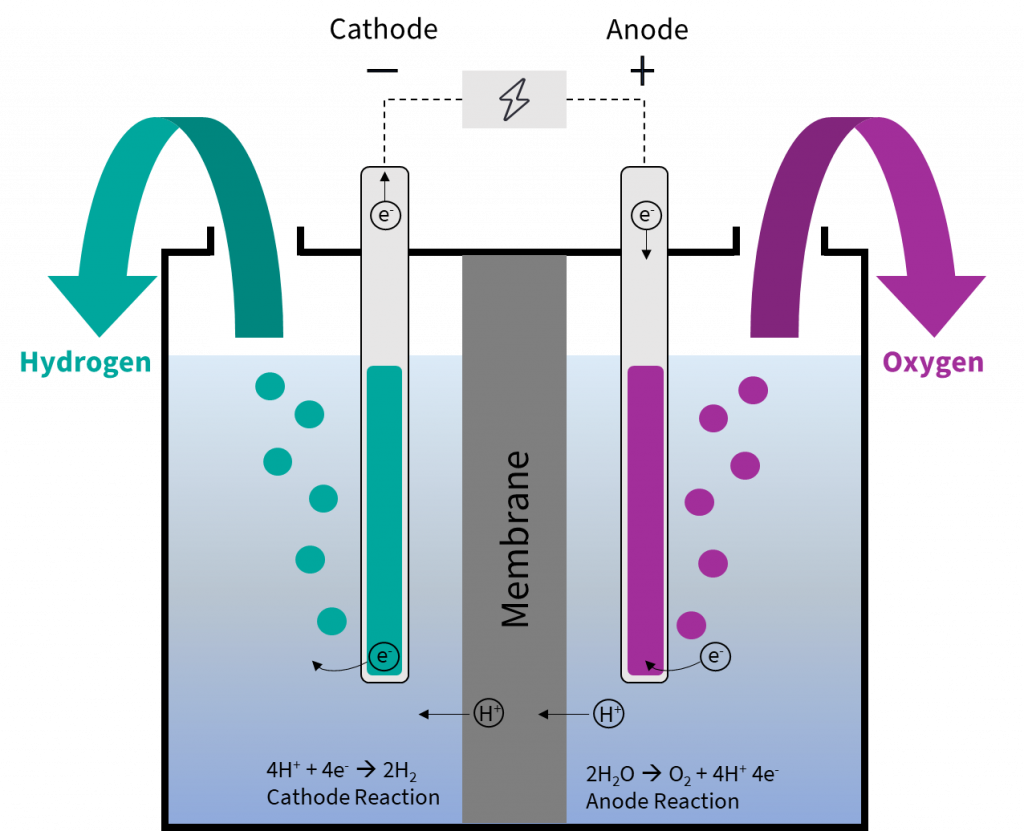
Water electrolysis explained the basis for most PowertoX processes PtX Hub
Salt Vs Baking Soda Electrolysis To do this experiment, you will need the following items: Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Some examples of electrolytes you. What electrolyte should i use in the water? I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. To do this experiment, you will need the following items: Mainly anything that contains ions (i.e. Electrolytes) is great to use in the electrolysis of water. Separating hydrogen and oxygen from water is done through electrolysis. Table salt (kosher salt is best since it’s.
From www.momswhothink.com
Baking Soda vs. Baking Powder There Really Is A Difference! Salt Vs Baking Soda Electrolysis Mainly anything that contains ions (i.e. Electrolytes) is great to use in the electrolysis of water. Separating hydrogen and oxygen from water is done through electrolysis. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. To do this experiment, you will need the following items:. Salt Vs Baking Soda Electrolysis.
From giozpojdj.blob.core.windows.net
Baking Soda Equation at Zachary Chatterton blog Salt Vs Baking Soda Electrolysis To do this experiment, you will need the following items: Some examples of electrolytes you. Separating hydrogen and oxygen from water is done through electrolysis. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. What electrolyte should i use in the water? Mainly anything that. Salt Vs Baking Soda Electrolysis.
From blog.thepipingmart.com
How to Remove Rust with Electrolysis An Overview Salt Vs Baking Soda Electrolysis I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. To do this experiment, you will need the following items: Some examples of electrolytes you. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. What electrolyte should i use in the water? Separating hydrogen and oxygen from water is done through electrolysis.. Salt Vs Baking Soda Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes PtX Hub Salt Vs Baking Soda Electrolysis In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Separating hydrogen and oxygen from water is done through electrolysis. Mainly anything that contains ions (i.e. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. What electrolyte should i use in the. Salt Vs Baking Soda Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Salt Vs Baking Soda Electrolysis To do this experiment, you will need the following items: What electrolyte should i use in the water? Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Separating hydrogen and oxygen. Salt Vs Baking Soda Electrolysis.
From www.pinterest.com.mx
Pin on STEM Salt Vs Baking Soda Electrolysis What electrolyte should i use in the water? Table salt (kosher salt is best since it’s. Electrolytes) is great to use in the electrolysis of water. To do this experiment, you will need the following items: Some examples of electrolytes you. Mainly anything that contains ions (i.e. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking.. Salt Vs Baking Soda Electrolysis.
From www.slideserve.com
PPT Electrolysis of salt 3 PowerPoint Presentation, free download ID4639459 Salt Vs Baking Soda Electrolysis Table salt (kosher salt is best since it’s. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Electrolytes) is great to use in the electrolysis of water. Mainly anything that contains ions (i.e. Some examples of electrolytes you. What electrolyte should i use in the. Salt Vs Baking Soda Electrolysis.
From inzest9ycmaterialdb.z21.web.core.windows.net
Baking Soda For Electrolysis Salt Vs Baking Soda Electrolysis To do this experiment, you will need the following items: Separating hydrogen and oxygen from water is done through electrolysis. Table salt (kosher salt is best since it’s. Electrolytes) is great to use in the electrolysis of water. Some examples of electrolytes you. In this demonstration, students investigate the idea that energy from a battery can be used to drive. Salt Vs Baking Soda Electrolysis.
From sodiumbicarbonate.com
Baking Soda vs Baking Powder What the Differences Are Sodium Bicarbonate Salt Vs Baking Soda Electrolysis What electrolyte should i use in the water? Mainly anything that contains ions (i.e. Separating hydrogen and oxygen from water is done through electrolysis. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. To do this experiment, you will need the following items: Some examples. Salt Vs Baking Soda Electrolysis.
From dbdalrymplesibships.z21.web.core.windows.net
Electrolysis With Baking Soda Salt Vs Baking Soda Electrolysis Some examples of electrolytes you. Electrolytes) is great to use in the electrolysis of water. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. To do this experiment, you will need the following items: Mainly anything that contains ions (i.e. What electrolyte should i use. Salt Vs Baking Soda Electrolysis.
From philschatz.com
Electrolysis · Chemistry Salt Vs Baking Soda Electrolysis Some examples of electrolytes you. Mainly anything that contains ions (i.e. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. Table salt (kosher salt is best since it’s. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Electrolytes) is great. Salt Vs Baking Soda Electrolysis.
From www.youtube.com
Electrolysis of Salt Solution at Home in Under 1 minute to produce Hydrogen and Chlorine YouTube Salt Vs Baking Soda Electrolysis I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Some examples of electrolytes you. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. What electrolyte should i use in the water? Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\). Salt Vs Baking Soda Electrolysis.
From www.youtube.com
Washing soda Acids, bases, and salts Chemistry Khan Academy YouTube Salt Vs Baking Soda Electrolysis Electrolytes) is great to use in the electrolysis of water. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. To do this experiment, you will need the following items: Table salt (kosher salt is best since it’s. What electrolyte should i use in the water?. Salt Vs Baking Soda Electrolysis.
From dbdalrymplesibships.z21.web.core.windows.net
Electrolysis With Baking Soda Salt Vs Baking Soda Electrolysis Separating hydrogen and oxygen from water is done through electrolysis. Electrolytes) is great to use in the electrolysis of water. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. To do. Salt Vs Baking Soda Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson Salt Vs Baking Soda Electrolysis Table salt (kosher salt is best since it’s. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Mainly anything that contains ions (i.e. Electrolytes) is great to use in the electrolysis of water. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not. Salt Vs Baking Soda Electrolysis.
From www.petes-tools.com
Uses for Sodium Bicarbonate or Baking Soda You Might Be Surprised Salt Vs Baking Soda Electrolysis Some examples of electrolytes you. Mainly anything that contains ions (i.e. Table salt (kosher salt is best since it’s. Electrolytes) is great to use in the electrolysis of water. What electrolyte should i use in the water? I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. In this demonstration, students investigate the idea that energy from. Salt Vs Baking Soda Electrolysis.
From sensorex.com
Electroplating The Process & Uses in Liquid Analysis Explained Sensorex Salt Vs Baking Soda Electrolysis Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. To do this experiment, you will need the following items: I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Table salt (kosher salt is best since it’s. Electrolytes) is great to use in the electrolysis of water. In this demonstration, students investigate. Salt Vs Baking Soda Electrolysis.
From studylibraryintroit.z14.web.core.windows.net
Electrolysis With Baking Soda Salt Vs Baking Soda Electrolysis Some examples of electrolytes you. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. Mainly anything that contains ions (i.e. To do this experiment, you will need the following items: Separating. Salt Vs Baking Soda Electrolysis.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Salt Vs Baking Soda Electrolysis Separating hydrogen and oxygen from water is done through electrolysis. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Electrolytes) is great to use in the electrolysis of water. Table salt (kosher salt is best since it’s. Mainly anything that contains ions (i.e. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\),. Salt Vs Baking Soda Electrolysis.
From www.vedaoils.com
Epsom Salt Vs Baking Soda Different Advantages & Disadvantages VedaOils Salt Vs Baking Soda Electrolysis I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. To do this experiment, you will need the following items: What electrolyte should i use in the water? Mainly anything that contains ions (i.e. Electrolytes) is great to use in the electrolysis of water. Table salt (kosher salt is best since it’s. In this demonstration, students investigate. Salt Vs Baking Soda Electrolysis.
From magicgouveiaswasher.z21.web.core.windows.net
Can You Use Baking Soda For Electrolysis Salt Vs Baking Soda Electrolysis Mainly anything that contains ions (i.e. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Some examples of electrolytes you. What electrolyte should i use in the water? Electrolytes) is great. Salt Vs Baking Soda Electrolysis.
From www.teachoo.com
Chemicals from Common Salt Caustic Soda, Bleaching Powder, Baking Salt Vs Baking Soda Electrolysis Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. Separating hydrogen and oxygen from water is done through electrolysis. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Table salt (kosher salt is best since it’s. Some examples of electrolytes. Salt Vs Baking Soda Electrolysis.
From www.pinterest.com
Electrolysis Chemical changes, Electrochemistry, Energy Salt Vs Baking Soda Electrolysis I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Table salt (kosher salt is best since it’s. Separating hydrogen and oxygen from water is done through electrolysis. Electrolytes) is great to use in the electrolysis of water. To do this experiment, you will need the following items: Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\),. Salt Vs Baking Soda Electrolysis.
From www.difference.wiki
Soda Crystals vs. Baking Soda What’s the Difference? Salt Vs Baking Soda Electrolysis Mainly anything that contains ions (i.e. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. What electrolyte should i use in the water? Electrolytes) is great to use in the electrolysis of water. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate. Salt Vs Baking Soda Electrolysis.
From www.youtube.com
Electrolysis of Salt Solutions YouTube Salt Vs Baking Soda Electrolysis Mainly anything that contains ions (i.e. What electrolyte should i use in the water? Some examples of electrolytes you. To do this experiment, you will need the following items: Table salt (kosher salt is best since it’s. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. Separating hydrogen and oxygen from water is done through. Salt Vs Baking Soda Electrolysis.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Salt Vs Baking Soda Electrolysis To do this experiment, you will need the following items: Separating hydrogen and oxygen from water is done through electrolysis. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. What electrolyte should. Salt Vs Baking Soda Electrolysis.
From www.flchemicals.com
Washing Soda vs Baking Soda What's The Difference? Fondland Salt Vs Baking Soda Electrolysis To do this experiment, you will need the following items: Electrolytes) is great to use in the electrolysis of water. What electrolyte should i use in the water? Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Table salt (kosher salt is. Salt Vs Baking Soda Electrolysis.
From www.youtube.com
3.6 ACIDIC, BASIC SALTS, ELECTROLYSIS OF BRINE SOLUTION E/M,T/M YouTube Salt Vs Baking Soda Electrolysis In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Mainly anything that contains ions (i.e. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. Some examples of electrolytes you. I was thinking of using magnesium sulfate (epsom salt) or sodium. Salt Vs Baking Soda Electrolysis.
From www.youtube.com
chemicals from common saltCaustic sodawashing sodaBaking soda YouTube Salt Vs Baking Soda Electrolysis Separating hydrogen and oxygen from water is done through electrolysis. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. Some examples of electrolytes you. Electrolytes) is great to use in the electrolysis of water. In this demonstration, students investigate the idea that. Salt Vs Baking Soda Electrolysis.
From www.dreamstime.com
The Electrolysis of Sodium Chloride. Vector Illustration Stock Vector Illustration of cathode Salt Vs Baking Soda Electrolysis Electrolytes) is great to use in the electrolysis of water. Separating hydrogen and oxygen from water is done through electrolysis. Table salt (kosher salt is best since it’s. Some examples of electrolytes you. To do this experiment, you will need the following items: In this demonstration, students investigate the idea that energy from a battery can be used to drive. Salt Vs Baking Soda Electrolysis.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets, videos) Salt Vs Baking Soda Electrolysis What electrolyte should i use in the water? Table salt (kosher salt is best since it’s. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. Some examples of electrolytes you. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Mainly anything that contains ions (i.e. Electrolytes) is great to use in. Salt Vs Baking Soda Electrolysis.
From www.chemistrylearner.com
Analytical Chemistry Chemistry Learner Salt Vs Baking Soda Electrolysis What electrolyte should i use in the water? Separating hydrogen and oxygen from water is done through electrolysis. To do this experiment, you will need the following items: Mainly anything that contains ions (i.e. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate. Salt Vs Baking Soda Electrolysis.
From sciencenotes.org
How to Turn Baking Soda Into Washing Soda (Sodium Bicarbonate to Sodium Carbonate) Salt Vs Baking Soda Electrolysis Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. Table salt (kosher salt is best since it’s. I was thinking of using magnesium sulfate (epsom salt) or sodium bicarbonate (baking. Some examples of electrolytes you. What electrolyte should i use in the water? Mainly anything that contains ions (i.e. In this demonstration, students investigate the. Salt Vs Baking Soda Electrolysis.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Salt Vs Baking Soda Electrolysis Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\), and. In this demonstration, students investigate the idea that energy from a battery can be used to drive a chemical reaction that does not happen. Separating hydrogen and oxygen from water is done through electrolysis. Electrolytes) is great to use in the electrolysis of water. I was. Salt Vs Baking Soda Electrolysis.
From www.youtube.com
Chemicals from Common Salt Class 10 Electrolysis of NaCl ChlorAlkali process Caustic soda Salt Vs Baking Soda Electrolysis Electrolytes) is great to use in the electrolysis of water. To do this experiment, you will need the following items: What electrolyte should i use in the water? Some examples of electrolytes you. Mainly anything that contains ions (i.e. Table salt (kosher salt is best since it’s. Some other ionic solids are \(\ce{cacl2}\), \(\ce{nh4cl}\), \(\ce{kbr}\), \(\ce{cuso4}\), \(\ce{nach3coo}\) (sodium acetate), \(\ce{caco3}\),. Salt Vs Baking Soda Electrolysis.