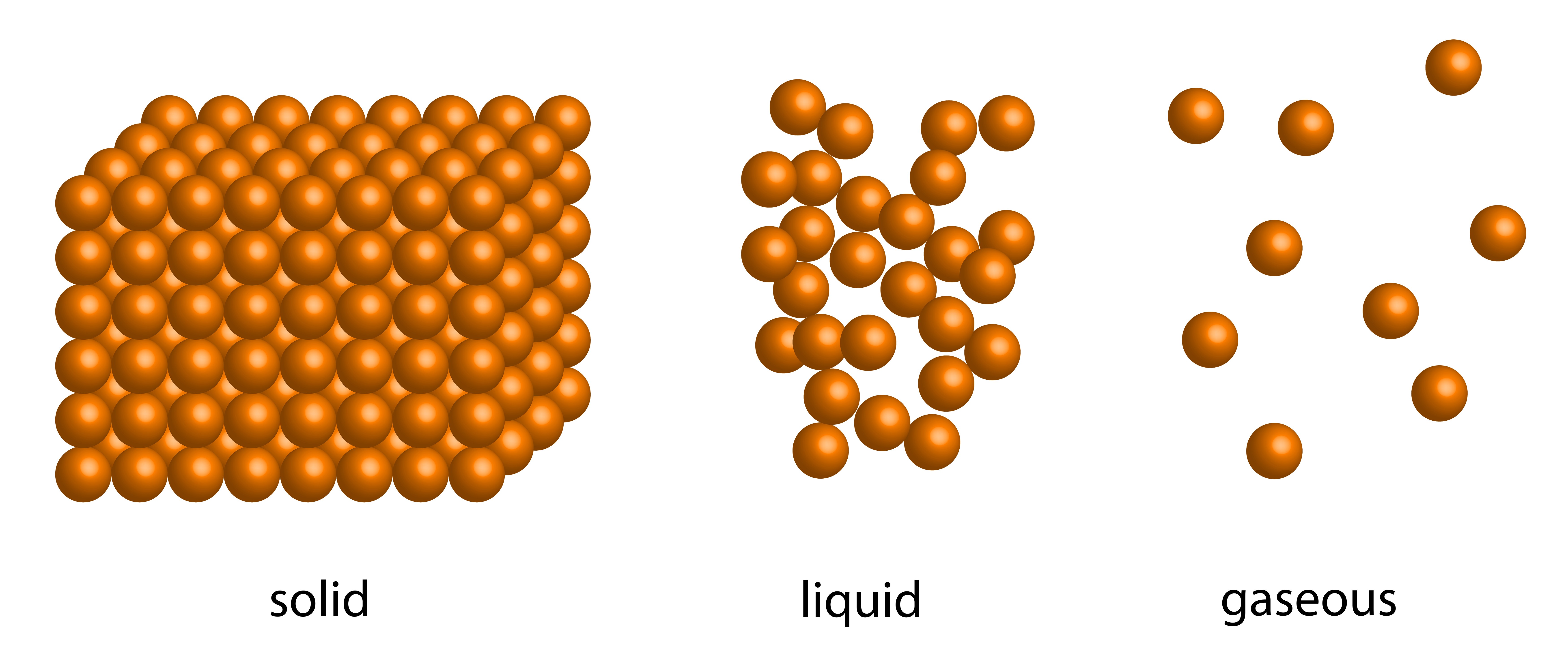Structural Differences Between Solids Liquids And Gases . The difference between solid, liquid and gas can be drawn clearly on the following grounds: a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. solids, liquids and gases. They do not spread out. They always take up the same amount of space. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. They do not flow like liquids. key differences between solid, liquid and gas. in general covalent bonds determine: liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. The change from solid to liquid usually does not significantly change the volume of a substance. figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at the molecular level, while. they keep their shape.
from guidewiringdietician.z5.web.core.windows.net
they keep their shape. in general covalent bonds determine: The difference between solid, liquid and gas can be drawn clearly on the following grounds: They always take up the same amount of space. They do not spread out. They do not flow like liquids. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. The change from solid to liquid usually does not significantly change the volume of a substance. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape.
3 States Of Matter Diagram
Structural Differences Between Solids Liquids And Gases They do not spread out. solids, liquids and gases. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at the molecular level, while. They do not spread out. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. in general covalent bonds determine: a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. The change from solid to liquid usually does not significantly change the volume of a substance. They always take up the same amount of space. They do not flow like liquids. The difference between solid, liquid and gas can be drawn clearly on the following grounds: key differences between solid, liquid and gas. they keep their shape.
From diagramlibraryconjoin.z19.web.core.windows.net
Solid Liquid And Gas Diagram Structural Differences Between Solids Liquids And Gases figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at the molecular level, while. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. they keep their shape. solids, liquids and gases. They do not flow like liquids. key differences between solid, liquid and gas. They. Structural Differences Between Solids Liquids And Gases.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Structural Differences Between Solids Liquids And Gases They always take up the same amount of space. The difference between solid, liquid and gas can be drawn clearly on the following grounds: They do not flow like liquids. they keep their shape. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. a solid has definite volume and shape,. Structural Differences Between Solids Liquids And Gases.
From newsela.com
There are three phases of matter solid, liquid and gas Structural Differences Between Solids Liquids And Gases In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. They always take up the same amount of space. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. figures \(\pageindex{3}\) and \(\pageindex{4}\) show. Structural Differences Between Solids Liquids And Gases.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Structural Differences Between Solids Liquids And Gases They always take up the same amount of space. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. solids, liquids. Structural Differences Between Solids Liquids And Gases.
From guidewiringdietician.z5.web.core.windows.net
3 States Of Matter Diagram Structural Differences Between Solids Liquids And Gases They do not spread out. in general covalent bonds determine: a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. They do not flow like liquids. liquids expand for the same reason as solids, but because the bonds between separate. Structural Differences Between Solids Liquids And Gases.
From brainly.in
difference between solid liquid and gas on the basis of their physical Structural Differences Between Solids Liquids And Gases liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. The change from solid to liquid usually does not significantly change the volume of a substance. solids, liquids and gases. they keep their shape. In a solid like this brick, the particles are regularly arranged touching their neighbours. Structural Differences Between Solids Liquids And Gases.
From www.pinterest.com
Difference between Solid, Liquid and Gas in Tabular form Solid liquid Structural Differences Between Solids Liquids And Gases in general covalent bonds determine: a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. They always take up the same amount of space. they keep their shape. solids, liquids and gases. The change from solid to liquid usually. Structural Differences Between Solids Liquids And Gases.
From exominksh.blob.core.windows.net
Solid Liquid Gas Difference at Jo Davis blog Structural Differences Between Solids Liquids And Gases They always take up the same amount of space. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at. Structural Differences Between Solids Liquids And Gases.
From stock.adobe.com
states of matter solids liquids and gases. Matter appears in three Structural Differences Between Solids Liquids And Gases In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. They always take up the same amount of space. figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at the molecular level, while. They do not flow like liquids. The difference between solid, liquid and gas can be drawn. Structural Differences Between Solids Liquids And Gases.
From johnwest.edublogs.org
Solids liquids and gasses, a story in three parts. Science Connected Structural Differences Between Solids Liquids And Gases liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. solids, liquids and gases. They do not flow like liquids. The change from solid to liquid usually does not significantly change the volume of a substance. They always take up the same amount of space. a solid has. Structural Differences Between Solids Liquids And Gases.
From learningideasgradesk-8.blogspot.com
Learning Ideas Grades K8 Simple and Compound Machines Venn Diagram Structural Differences Between Solids Liquids And Gases figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at the molecular level, while. The change from solid to liquid usually does not significantly change the volume of a substance. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. key differences between solid, liquid and gas. The. Structural Differences Between Solids Liquids And Gases.
From learningmagicprorogues.z13.web.core.windows.net
Matter Chart Solid Liquid Gas Structural Differences Between Solids Liquids And Gases They always take up the same amount of space. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. they keep their shape. The change from solid to liquid usually does not significantly change the volume of a substance. liquids. Structural Differences Between Solids Liquids And Gases.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Structural Differences Between Solids Liquids And Gases liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. in general covalent bonds determine: The change from solid to liquid usually does not significantly change the volume of a substance. They do not spread out. a solid has definite volume and shape, a liquid has a definite. Structural Differences Between Solids Liquids And Gases.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Structural Differences Between Solids Liquids And Gases In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. The change from solid to liquid usually does not significantly change the volume of a substance. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. key differences between solid, liquid and. Structural Differences Between Solids Liquids And Gases.
From igcsechemistryrevision.weebly.com
All Categories iGCSE CHEMISTRY REVISION HELP Structural Differences Between Solids Liquids And Gases a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. they keep their shape. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. The difference between solid, liquid and gas. Structural Differences Between Solids Liquids And Gases.
From lessoncampuscontessa.z4.web.core.windows.net
Three States Of Matter Solid Liquid And Gas Structural Differences Between Solids Liquids And Gases they keep their shape. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. The change from solid to liquid usually does not significantly change the volume of a substance. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. solids,. Structural Differences Between Solids Liquids And Gases.
From schematicliblatinas77.z22.web.core.windows.net
Diagram Of Particles In A Solid Structural Differences Between Solids Liquids And Gases in general covalent bonds determine: a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. They do not spread out. They. Structural Differences Between Solids Liquids And Gases.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube Structural Differences Between Solids Liquids And Gases key differences between solid, liquid and gas. They do not spread out. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. They always. Structural Differences Between Solids Liquids And Gases.
From www.toppr.com
Difference Between Solid Liquid and Gas with Definitions Structural Differences Between Solids Liquids And Gases They do not flow like liquids. in general covalent bonds determine: They always take up the same amount of space. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. The change. Structural Differences Between Solids Liquids And Gases.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The Structural Differences Between Solids Liquids And Gases The change from solid to liquid usually does not significantly change the volume of a substance. solids, liquids and gases. They do not spread out. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. a solid has definite volume and shape, a liquid has a definite volume. Structural Differences Between Solids Liquids And Gases.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Structural Differences Between Solids Liquids And Gases liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. The difference between solid, liquid and gas can be drawn clearly on the following grounds: a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite. Structural Differences Between Solids Liquids And Gases.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Structural Differences Between Solids Liquids And Gases The change from solid to liquid usually does not significantly change the volume of a substance. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. they keep their shape. They do not spread out. figures \(\pageindex{3}\) and \(\pageindex{4}\) show. Structural Differences Between Solids Liquids And Gases.
From exominksh.blob.core.windows.net
Solid Liquid Gas Difference at Jo Davis blog Structural Differences Between Solids Liquids And Gases In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. They do not spread out. The difference between solid, liquid and gas can be drawn clearly on the following grounds: in general covalent bonds determine: they keep their shape. solids, liquids and gases. liquids expand for the same reason. Structural Differences Between Solids Liquids And Gases.
From byjus.com
Draw a sketch to show the arrangement of particles in solid, liquid and Structural Differences Between Solids Liquids And Gases solids, liquids and gases. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. They always take up the same amount of space. in general covalent bonds determine: The difference between solid, liquid and gas can be drawn clearly on the following grounds: a solid has definite. Structural Differences Between Solids Liquids And Gases.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison Structural Differences Between Solids Liquids And Gases key differences between solid, liquid and gas. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. They do not spread out. figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at the molecular level, while. The. Structural Differences Between Solids Liquids And Gases.
From slgas03.blogspot.com
solid,liquid and gas States of Matter Structural Differences Between Solids Liquids And Gases The change from solid to liquid usually does not significantly change the volume of a substance. figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at the molecular level, while. they keep their shape. in general covalent bonds determine: a solid has definite volume and shape, a liquid has a definite volume but. Structural Differences Between Solids Liquids And Gases.
From www.jove.com
11340.jpg Structural Differences Between Solids Liquids And Gases liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. solids, liquids and gases. They do not spread out. The change from solid to liquid usually does not significantly change the volume of a substance. they keep their shape. They do not flow like liquids. In a solid. Structural Differences Between Solids Liquids And Gases.
From www.pinterest.com
Differences between Solids, Liquids & Gases Solid liquid gas, Biology Structural Differences Between Solids Liquids And Gases a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. They do not spread out. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. They do not flow like liquids. . Structural Differences Between Solids Liquids And Gases.
From www.tutorix.com
Give three characteristics of solid liquid and gas Tutorix Structural Differences Between Solids Liquids And Gases In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. The change from solid to liquid usually does not significantly change the volume of a substance. The difference between solid, liquid and gas can be drawn clearly on the following grounds: figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and. Structural Differences Between Solids Liquids And Gases.
From dxokdwwhh.blob.core.windows.net
What Are Differences Between Solids And Liquids at Jessica Mann blog Structural Differences Between Solids Liquids And Gases key differences between solid, liquid and gas. They do not flow like liquids. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at the molecular level, while.. Structural Differences Between Solids Liquids And Gases.
From www.pinterest.ph
Properties of Solids, Liquids, Gases Compared Teachoo Science Structural Differences Between Solids Liquids And Gases solids, liquids and gases. they keep their shape. The difference between solid, liquid and gas can be drawn clearly on the following grounds: They do not flow like liquids. The change from solid to liquid usually does not significantly change the volume of a substance. figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases. Structural Differences Between Solids Liquids And Gases.
From examplespedia.com
Difference between Solid Liquid and Gas ExamplesPedia Structural Differences Between Solids Liquids And Gases solids, liquids and gases. key differences between solid, liquid and gas. They do not flow like liquids. They do not spread out. they keep their shape. liquids expand for the same reason as solids, but because the bonds between separate molecules are usually less tight,. figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids,. Structural Differences Between Solids Liquids And Gases.
From www.ase.org.uk
Solids, liquids and gases Structural Differences Between Solids Liquids And Gases The difference between solid, liquid and gas can be drawn clearly on the following grounds: in general covalent bonds determine: a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. They always take up the same amount of space. solids,. Structural Differences Between Solids Liquids And Gases.
From jennybmooreo.blob.core.windows.net
Solid Liquid Gas Solution at jennybmooreo blog Structural Differences Between Solids Liquids And Gases They always take up the same amount of space. They do not flow like liquids. in general covalent bonds determine: key differences between solid, liquid and gas. They do not spread out. they keep their shape. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. The change from solid. Structural Differences Between Solids Liquids And Gases.
From www.dreamstime.com
States of Matter. Vector Circles Infographic Illustration Stock Vector Structural Differences Between Solids Liquids And Gases figures \(\pageindex{3}\) and \(\pageindex{4}\) show the differences among solids, liquids, and gases at the molecular level, while. They always take up the same amount of space. in general covalent bonds determine: In a solid like this brick, the particles are regularly arranged touching their neighbours and move only. liquids expand for the same reason as solids, but. Structural Differences Between Solids Liquids And Gases.