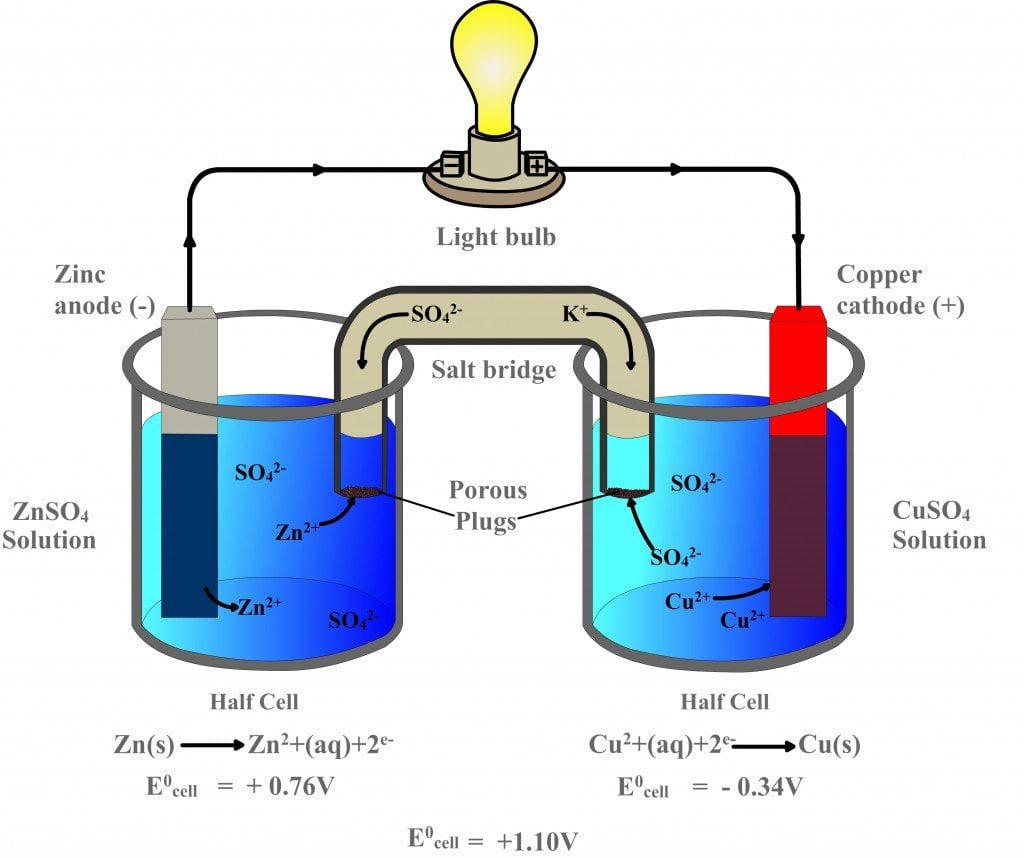
Galvanic Cell Vs A Battery . For a daniell cell, the cell potential is:. In contrast, a fuel cell is a. a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. a battery is an electrochemical cell or series of cells that produces an electric current.
from www.scienceabc.com
the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. In contrast, a fuel cell is a. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. For a daniell cell, the cell potential is:. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a battery is an electrochemical cell or series of cells that produces an electric current. a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity.
Galvanic Cell Definition, Diagram And Working
Galvanic Cell Vs A Battery the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. In contrast, a fuel cell is a. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: a battery is an electrochemical cell or series of cells that produces an electric current. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. For a daniell cell, the cell potential is:. the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Galvanic Cell Vs A Battery For a daniell cell, the cell potential is:. the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. a. Galvanic Cell Vs A Battery.
From flatworldknowledge.lardbucket.org
Commercial Galvanic Cells Galvanic Cell Vs A Battery a battery is an electrochemical cell or series of cells that produces an electric current. the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom. Galvanic Cell Vs A Battery.
From www.youtube.com
Electrochemistry Galvanic/Voltaic Cell Battery Made Super Simple! MCAT Galvanic Cell Vs A Battery the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. For a daniell cell, the cell potential is:. a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. the galvanic cell, or called. Galvanic Cell Vs A Battery.
From www.wisegeek.com
What is the Galvanic Cell? (with picture) Galvanic Cell Vs A Battery it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. In contrast, a fuel cell is a. (i) the difference in the lattice cohesive. Galvanic Cell Vs A Battery.
From facts.net
9 Enigmatic Facts About Galvanic Cell Galvanic Cell Vs A Battery For a daniell cell, the cell potential is:. a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. a battery is an electrochemical cell or series of cells that produces an electric current. the galvanic cell, or called voltaic cell, is an electrochemical cell. Galvanic Cell Vs A Battery.
From edurev.in
Daniel Cell Or Galvanic Cell Class 12 Notes EduRev Galvanic Cell Vs A Battery For a daniell cell, the cell potential is:. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. the galvanic cell, or. Galvanic Cell Vs A Battery.
From saylordotorg.github.io
Commercial Galvanic Cells Galvanic Cell Vs A Battery it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. In contrast, a fuel cell is a. a battery is an electrochemical cell. Galvanic Cell Vs A Battery.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Galvanic Cell Vs A Battery a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: (i) the difference in the lattice cohesive energies of the bulk. Galvanic Cell Vs A Battery.
From exodgcmgz.blob.core.windows.net
Fuel Galvanic Cells at Sean Isom blog Galvanic Cell Vs A Battery a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. a battery is an electrochemical cell or series of cells that produces an electric current. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to. Galvanic Cell Vs A Battery.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Galvanic Cell Vs A Battery the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: In contrast, a fuel cell is a. a battery is an electrochemical cell. Galvanic Cell Vs A Battery.
From saylordotorg.github.io
Commercial Galvanic Cells Galvanic Cell Vs A Battery (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. a battery is an electrochemical cell or. Galvanic Cell Vs A Battery.
From biochemreview.weebly.com
Galvanic cell BIOChemReview Galvanic Cell Vs A Battery (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. a battery is an electrochemical cell or series of cells that produces an electric current. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical. Galvanic Cell Vs A Battery.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE Galvanic Cell Vs A Battery it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. the e o cell value is positive for a galvanic. Galvanic Cell Vs A Battery.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle Galvanic Cell Vs A Battery (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: the e o cell value is. Galvanic Cell Vs A Battery.
From www.electricity-magnetism.org
Voltage of Electric Batteries Cell Voltage Electricity Galvanic Cell Vs A Battery In contrast, a fuel cell is a. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: (i) the difference in the lattice cohesive. Galvanic Cell Vs A Battery.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Galvanic Cell Vs A Battery the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively. Galvanic Cell Vs A Battery.
From www.vrogue.co
Grade 12 A Science Galvanic And Electrolytic Cells El vrogue.co Galvanic Cell Vs A Battery In contrast, a fuel cell is a. a battery is an electrochemical cell or series of cells that produces an electric current. For a daniell cell, the cell potential is:. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. . Galvanic Cell Vs A Battery.
From courses.lumenlearning.com
Galvanic Cells Chemistry Galvanic Cell Vs A Battery the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: the galvanic cell, or called voltaic cell, is an electrochemical cell that. Galvanic Cell Vs A Battery.
From classnotes.org.in
Electrochemical Cells Chemistry, Class 12, Electro Chemistry Galvanic Cell Vs A Battery it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. a battery is an electrochemical cell or series of cells that produces. Galvanic Cell Vs A Battery.
From www.vrogue.co
Electric Battery Symbol Galvanic Cell Electricity Cir vrogue.co Galvanic Cell Vs A Battery In contrast, a fuel cell is a. For a daniell cell, the cell potential is:. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are. Galvanic Cell Vs A Battery.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download Galvanic Cell Vs A Battery a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: For a daniell cell, the cell potential is:. a battery. Galvanic Cell Vs A Battery.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Galvanic Cell Vs A Battery the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. a battery is an electrochemical cell or series of cells that produces an electric current. a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to. Galvanic Cell Vs A Battery.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER Galvanic Cell Vs A Battery a battery is an electrochemical cell or series of cells that produces an electric current. For a daniell cell, the cell potential is:. In contrast, a fuel cell is a. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: a battery (storage. Galvanic Cell Vs A Battery.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Galvanic Cell Vs A Battery (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. In contrast, a fuel cell is a. the e o. Galvanic Cell Vs A Battery.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry Galvanic Cell Vs A Battery a battery is an electrochemical cell or series of cells that produces an electric current. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: In contrast, a fuel cell is a. a battery (storage cell) is a galvanic cell (or a series. Galvanic Cell Vs A Battery.
From www.javatpoint.com
Difference Between Galvanic Cells and Electrolytic Cells javatpoint Galvanic Cell Vs A Battery For a daniell cell, the cell potential is:. a battery is an electrochemical cell or series of cells that produces an electric current. In contrast, a fuel cell is a. the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. it is shown that, for simple. Galvanic Cell Vs A Battery.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram Galvanic Cell Vs A Battery the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. In contrast, a fuel cell is a. For a daniell cell, the cell potential is:. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are. Galvanic Cell Vs A Battery.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Galvanic Cell Vs A Battery a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. In contrast, a fuel cell is a. For a daniell cell, the cell potential is:.. Galvanic Cell Vs A Battery.
From www.pinterest.com.au
Table of ContentsWhat is a battery? Understanding galvanic cells Galvanic Cell Vs A Battery the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and. Galvanic Cell Vs A Battery.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Galvanic Cell Vs A Battery it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: In contrast, a fuel cell is a. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a battery (storage cell) is a. Galvanic Cell Vs A Battery.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Galvanic Cell Vs A Battery a battery (storage cell) is a galvanic cell (or a series of galvanic cells) that contains all the reactants needed to produce electricity. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. In contrast, a fuel cell is a. . Galvanic Cell Vs A Battery.
From www.vrogue.co
Electric Battery Symbol Galvanic Cell Electricity Cir vrogue.co Galvanic Cell Vs A Battery (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. the e o cell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. it is shown that, for simple galvanic cells or. Galvanic Cell Vs A Battery.
From jesse-has-lozano.blogspot.com
Explain the Difference Between a Galvanic Cell and Electrolytic Cell Galvanic Cell Vs A Battery (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. For a daniell cell, the cell potential is:. it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are. Galvanic Cell Vs A Battery.
From www.peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Galvanic Cell Vs A Battery For a daniell cell, the cell potential is:. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference in. In contrast, a fuel cell is a. a battery is an electrochemical cell or series of cells that produces an electric current. . Galvanic Cell Vs A Battery.
From slidetodoc.com
Batteries and Galvanic Cells 1 Why Study Electrochemistry Galvanic Cell Vs A Battery it is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful contributions to the electrical energy are relevant: For a daniell cell, the cell potential is:. (i) the difference in the lattice cohesive energies of the bulk metals, reflecting metallic and covalent bonding and accounting for the atom transfer, and (ii) the difference. Galvanic Cell Vs A Battery.