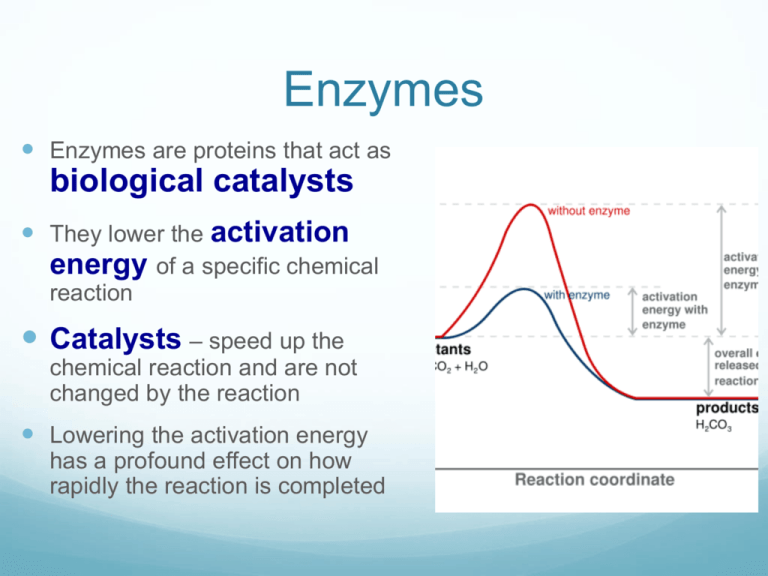
How Can A Catalyst Lower The Activation Energy . catalysts and activation energy. — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. catalyst lower activation energy is: By altering the reaction's transition state, a catalyst lowers the activation energy. One possible way of doing this. The reaction then goes through a different pathway/mechanism than. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. A catalyst lowers the activation energy of a chemical reaction. To increase the rate of a reaction you need to increase the number of successful collisions. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. a catalyst lowers the activation energy by changing the transition state of the reaction. — effect of enzymes and catalysts.
from studylib.net
a catalyst lowers the activation energy by changing the transition state of the reaction. A catalyst lowers the activation energy of a chemical reaction. Catalysts are defined as substances that participate in a chemical reaction but. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. — effect of enzymes and catalysts. By altering the reaction's transition state, a catalyst lowers the activation energy. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. catalyst lower activation energy is:
Enzymes Lower Activation Energy
How Can A Catalyst Lower The Activation Energy By altering the reaction's transition state, a catalyst lowers the activation energy. — effect of enzymes and catalysts. a catalyst lowers the activation energy by changing the transition state of the reaction. To increase the rate of a reaction you need to increase the number of successful collisions. The reaction then goes through a different pathway/mechanism than. A catalyst lowers the activation energy of a chemical reaction. By altering the reaction's transition state, a catalyst lowers the activation energy. One possible way of doing this. catalyst lower activation energy is: catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalysts and activation energy. Catalysts are defined as substances that participate in a chemical reaction but. — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and.
From byjus.com
How does catalyst affect activation energy? How Can A Catalyst Lower The Activation Energy a catalyst lowers the activation energy by changing the transition state of the reaction. To increase the rate of a reaction you need to increase the number of successful collisions. By altering the reaction's transition state, a catalyst lowers the activation energy. — one possible way of doing this is to provide an alternative way for the reaction. How Can A Catalyst Lower The Activation Energy.
From 2012books.lardbucket.org
Catalysis How Can A Catalyst Lower The Activation Energy Catalysts are defined as substances that participate in a chemical reaction but. By altering the reaction's transition state, a catalyst lowers the activation energy. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower.. How Can A Catalyst Lower The Activation Energy.
From www.slideserve.com
PPT Reaction PowerPoint Presentation ID1835084 How Can A Catalyst Lower The Activation Energy — effect of enzymes and catalysts. a catalyst lowers the activation energy by changing the transition state of the reaction. catalysts and activation energy. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. To increase the rate of a reaction you need to increase the number of. How Can A Catalyst Lower The Activation Energy.
From www.slideserve.com
PPT Enzyme Biological Catalyst (Part ii) PowerPoint Presentation How Can A Catalyst Lower The Activation Energy A catalyst lowers the activation energy of a chemical reaction. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. — effect of enzymes and catalysts. catalysts and activation energy. To increase the rate of a reaction you need to increase the number. How Can A Catalyst Lower The Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii How Can A Catalyst Lower The Activation Energy — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. catalysts and activation energy. — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. The reaction then goes through a different pathway/mechanism than. catalyst lower activation. How Can A Catalyst Lower The Activation Energy.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished How Can A Catalyst Lower The Activation Energy catalyst lower activation energy is: — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. — effect of enzymes and catalysts. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. A catalyst lowers the activation energy of a chemical reaction. Catalysts. How Can A Catalyst Lower The Activation Energy.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited How Can A Catalyst Lower The Activation Energy By altering the reaction's transition state, a catalyst lowers the activation energy. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalyst lower activation energy is: — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. One possible way of doing this. Catalysts are defined as. How Can A Catalyst Lower The Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph How Can A Catalyst Lower The Activation Energy Catalysts are defined as substances that participate in a chemical reaction but. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. a catalyst lowers the activation energy by changing the transition state of the reaction. catalyst lower activation energy is: — effect of enzymes and catalysts. . How Can A Catalyst Lower The Activation Energy.
From studylib.net
Enzymes Lower Activation Energy How Can A Catalyst Lower The Activation Energy catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalyst lower activation energy is: By altering the reaction's transition state, a catalyst lowers the activation energy. One possible way of doing this. A catalyst lowers the activation energy of a chemical reaction. — catalysts permit an alternate mechanism for the reactants to become products,. How Can A Catalyst Lower The Activation Energy.
From telegra.ph
Catalyst activation energy diagram of enzymes Telegraph How Can A Catalyst Lower The Activation Energy To increase the rate of a reaction you need to increase the number of successful collisions. The reaction then goes through a different pathway/mechanism than. One possible way of doing this. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. — effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical. How Can A Catalyst Lower The Activation Energy.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation ID5736986 How Can A Catalyst Lower The Activation Energy a catalyst lowers the activation energy by changing the transition state of the reaction. Catalysts are defined as substances that participate in a chemical reaction but. The reaction then goes through a different pathway/mechanism than. A catalyst lowers the activation energy of a chemical reaction. catalyst lower activation energy is: — effect of enzymes and catalysts. . How Can A Catalyst Lower The Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free How Can A Catalyst Lower The Activation Energy By altering the reaction's transition state, a catalyst lowers the activation energy. — effect of enzymes and catalysts. One possible way of doing this. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. A catalyst lowers the activation energy of a chemical reaction. — one possible way of. How Can A Catalyst Lower The Activation Energy.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes How Can A Catalyst Lower The Activation Energy — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. catalysts and activation energy. — effect of enzymes and catalysts. By altering the reaction's transition state, a catalyst lowers the activation energy. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means. How Can A Catalyst Lower The Activation Energy.
From slideplayer.com
How Catalysts Work Main Concept ppt download How Can A Catalyst Lower The Activation Energy — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. To increase the rate of a reaction you need to increase the number of successful collisions. The reaction then goes through a different pathway/mechanism than. a catalyst lowers the activation energy by changing the transition state of the reaction. Catalysts. How Can A Catalyst Lower The Activation Energy.
From www.savemyexams.com
Lowering of Activation Energy College Board AP Biology Revision Notes How Can A Catalyst Lower The Activation Energy catalyst lower activation energy is: The reaction then goes through a different pathway/mechanism than. To increase the rate of a reaction you need to increase the number of successful collisions. By altering the reaction's transition state, a catalyst lowers the activation energy. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation. How Can A Catalyst Lower The Activation Energy.
From schematicbraginamh.z4.web.core.windows.net
Energy Diagram For Chemical Reaction How Can A Catalyst Lower The Activation Energy — effect of enzymes and catalysts. — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. The reaction then goes through a different pathway/mechanism than. catalyst lower activation energy is: — catalysts permit an alternate mechanism for the reactants to become products, with a. How Can A Catalyst Lower The Activation Energy.
From www.slideserve.com
PPT Chemical reactions and enzymes PowerPoint Presentation, free How Can A Catalyst Lower The Activation Energy — effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. The reaction then goes through a different pathway/mechanism than. One possible way of doing this. catalyst lower activation energy. How Can A Catalyst Lower The Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by How Can A Catalyst Lower The Activation Energy A catalyst lowers the activation energy of a chemical reaction. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalysts are defined as substances that participate in a chemical reaction but. The reaction then goes through a. How Can A Catalyst Lower The Activation Energy.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram How Can A Catalyst Lower The Activation Energy Catalysts are defined as substances that participate in a chemical reaction but. By altering the reaction's transition state, a catalyst lowers the activation energy. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. One possible way of doing this. — effect of enzymes and catalysts. catalysts and activation. How Can A Catalyst Lower The Activation Energy.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry How Can A Catalyst Lower The Activation Energy By altering the reaction's transition state, a catalyst lowers the activation energy. A catalyst lowers the activation energy of a chemical reaction. catalysts and activation energy. a catalyst lowers the activation energy by changing the transition state of the reaction. catalyst lower activation energy is: — one possible way of doing this is to provide an. How Can A Catalyst Lower The Activation Energy.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 How Can A Catalyst Lower The Activation Energy — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. Catalysts are defined as substances that participate in a chemical reaction but. To increase the rate of a reaction you need to increase the number of successful collisions. — effect of enzymes and catalysts. The reaction. How Can A Catalyst Lower The Activation Energy.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation How Can A Catalyst Lower The Activation Energy A catalyst lowers the activation energy of a chemical reaction. The reaction then goes through a different pathway/mechanism than. a catalyst lowers the activation energy by changing the transition state of the reaction. — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. — catalysts. How Can A Catalyst Lower The Activation Energy.
From dxoodakes.blob.core.windows.net
How Does An Enzyme Lower The Activation Energy Of A Chemical Reaction How Can A Catalyst Lower The Activation Energy The reaction then goes through a different pathway/mechanism than. — effect of enzymes and catalysts. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. By altering the reaction's transition state, a catalyst lowers the activation energy. catalyst lower activation energy is: Catalysts are defined as substances that participate. How Can A Catalyst Lower The Activation Energy.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free How Can A Catalyst Lower The Activation Energy catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. a catalyst lowers the activation energy by changing the transition state of the reaction. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. To increase the rate of a reaction you need to increase the number. How Can A Catalyst Lower The Activation Energy.
From www.energyfrontier.us
When Less Is More Energy Frontier Research Center How Can A Catalyst Lower The Activation Energy catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. a catalyst lowers the activation energy by changing the transition state of the reaction. — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. catalyst lower activation energy is: — catalysts. How Can A Catalyst Lower The Activation Energy.
From present5.com
Enzyme Structure classification and mechanism of action How Can A Catalyst Lower The Activation Energy One possible way of doing this. To increase the rate of a reaction you need to increase the number of successful collisions. The reaction then goes through a different pathway/mechanism than. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalyst lower activation energy is: A catalyst lowers the activation energy of a chemical reaction.. How Can A Catalyst Lower The Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation How Can A Catalyst Lower The Activation Energy — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. — effect of enzymes and catalysts. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalysts and activation energy. Catalysts are defined as substances that participate in a chemical reaction but.. How Can A Catalyst Lower The Activation Energy.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 How Can A Catalyst Lower The Activation Energy To increase the rate of a reaction you need to increase the number of successful collisions. A catalyst lowers the activation energy of a chemical reaction. One possible way of doing this. a catalyst lowers the activation energy by changing the transition state of the reaction. catalysts and activation energy. By altering the reaction's transition state, a catalyst. How Can A Catalyst Lower The Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of How Can A Catalyst Lower The Activation Energy By altering the reaction's transition state, a catalyst lowers the activation energy. One possible way of doing this. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. A catalyst lowers the activation energy of a chemical reaction. — effect of enzymes and catalysts. a catalyst lowers the activation. How Can A Catalyst Lower The Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation How Can A Catalyst Lower The Activation Energy By altering the reaction's transition state, a catalyst lowers the activation energy. One possible way of doing this. catalysts and activation energy. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. To increase the rate of a reaction you need to increase the number of successful collisions. a. How Can A Catalyst Lower The Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs How Can A Catalyst Lower The Activation Energy One possible way of doing this. — one possible way of doing this is to provide an alternative way for the reaction to happen which has a lower. By altering the reaction's transition state, a catalyst lowers the activation energy. catalysts and activation energy. The reaction then goes through a different pathway/mechanism than. a catalyst lowers the. How Can A Catalyst Lower The Activation Energy.
From exoajjasl.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction From 20 To 10 at How Can A Catalyst Lower The Activation Energy A catalyst lowers the activation energy of a chemical reaction. — effect of enzymes and catalysts. One possible way of doing this. Catalysts are defined as substances that participate in a chemical reaction but. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalyst lower activation energy is: catalysts and activation energy. . How Can A Catalyst Lower The Activation Energy.
From slideplayer.com
Chemical Reactions. ppt download How Can A Catalyst Lower The Activation Energy One possible way of doing this. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. By altering the reaction's transition state, a catalyst lowers the activation energy. a catalyst lowers the activation energy by changing the transition state of the reaction. — one possible way of doing this is to provide an alternative way. How Can A Catalyst Lower The Activation Energy.
From exoajjasl.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction From 20 To 10 at How Can A Catalyst Lower The Activation Energy catalysts and activation energy. catalyst lower activation energy is: A catalyst lowers the activation energy of a chemical reaction. Catalysts are defined as substances that participate in a chemical reaction but. a catalyst lowers the activation energy by changing the transition state of the reaction. One possible way of doing this. — catalysts permit an alternate. How Can A Catalyst Lower The Activation Energy.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) How Can A Catalyst Lower The Activation Energy To increase the rate of a reaction you need to increase the number of successful collisions. — effect of enzymes and catalysts. catalysts and activation energy. — catalysts permit an alternate mechanism for the reactants to become products, with a lower activation energy and. A catalyst lowers the activation energy of a chemical reaction. The reaction then. How Can A Catalyst Lower The Activation Energy.