How Are Photons Absorbed By Electrons . Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. The binding energy of the. For example, if a red photon of frequency \(f\). In photoelectric absorption, a photon disappears being absorbed by an atomic electron. The process results in ionization by subsequent ejection of the electron from the atom. The same interaction that admits this pushing and pulling is the. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon completely disappears. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave form to longitudinal wave form. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the atom i.e. The interaction occurs not with the. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy.
from www.youtube.com
The binding energy of the. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. For example, if a red photon of frequency \(f\). The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the atom i.e. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. The interaction occurs not with the. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The process results in ionization by subsequent ejection of the electron from the atom.
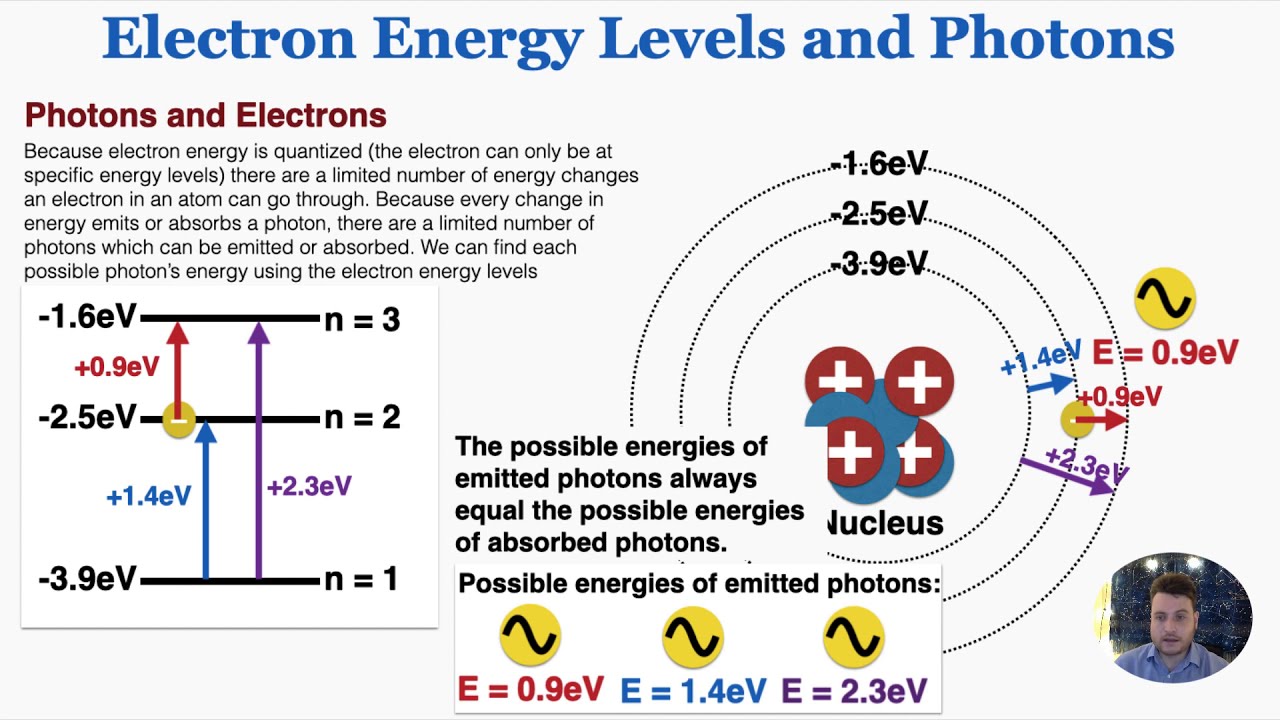
Electron Energy Levels and Photons IB Physics YouTube
How Are Photons Absorbed By Electrons In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon completely disappears. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave form to longitudinal wave form. The same interaction that admits this pushing and pulling is the. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The binding energy of the. For example, if a red photon of frequency \(f\). The interaction occurs not with the. The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the atom i.e. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon completely disappears. The process results in ionization by subsequent ejection of the electron from the atom. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. In photoelectric absorption, a photon disappears being absorbed by an atomic electron.
From chem.libretexts.org
Chapter 2.3 Atomic Spectra and Models of the Atom Chemistry LibreTexts How Are Photons Absorbed By Electrons The interaction occurs not with the. For example, if a red photon of frequency \(f\). The process results in ionization by subsequent ejection of the electron from the atom. The binding energy of the. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave form to longitudinal wave form. The energy of the. How Are Photons Absorbed By Electrons.
From www.pinterest.com
Light and photosynthetic pigments The lightdependent reactions How Are Photons Absorbed By Electrons Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. The same interaction that admits this pushing and pulling is the. For example, if a red photon of frequency \(f\). The energy of the liberated electron. How Are Photons Absorbed By Electrons.
From www.slideshare.net
Interaction of photons with matter How Are Photons Absorbed By Electrons In photoelectric absorption, a photon disappears being absorbed by an atomic electron. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. The interaction occurs not with the. Single atoms can absorb energy from a photon. How Are Photons Absorbed By Electrons.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps How Are Photons Absorbed By Electrons The binding energy of the. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave form. How Are Photons Absorbed By Electrons.
From courses.lumenlearning.com
The LightDependent Reactions of Photosynthesis Biology I How Are Photons Absorbed By Electrons Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the. How Are Photons Absorbed By Electrons.
From www.slideserve.com
PPT Matter and Particles of Light Quantum Theory PowerPoint How Are Photons Absorbed By Electrons The binding energy of the. The interaction occurs not with the. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. The process results in ionization by subsequent ejection of the electron from. How Are Photons Absorbed By Electrons.
From www.slideserve.com
PPT Interaction of electrons with matter Interaction of photons with How Are Photons Absorbed By Electrons Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. In the photoelectric absorption. How Are Photons Absorbed By Electrons.
From slideplayer.com
Chapter 8 Continuous Absorption ppt download How Are Photons Absorbed By Electrons For example, if a red photon of frequency \(f\). Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave. How Are Photons Absorbed By Electrons.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum How Are Photons Absorbed By Electrons In photoelectric absorption, a photon disappears being absorbed by an atomic electron. Charges absorb and emit photons, and that's how they experience the electromagnetic force. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. A. How Are Photons Absorbed By Electrons.
From study.com
Electron Transition Definition, Chart & Examples Lesson How Are Photons Absorbed By Electrons Charges absorb and emit photons, and that's how they experience the electromagnetic force. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave form to longitudinal wave form. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in. How Are Photons Absorbed By Electrons.
From rwu.pressbooks.pub
Chapter 12. Photosynthesis Introduction to Molecular and Cell Biology How Are Photons Absorbed By Electrons In photoelectric absorption, a photon disappears being absorbed by an atomic electron. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon completely disappears. For example, if a red photon of frequency \(f\). The interaction occurs not with the. Photons can also be produced when electrons that are bound in atoms by. How Are Photons Absorbed By Electrons.
From energywavetheory.com
What is Quantum? EWT How Are Photons Absorbed By Electrons The same interaction that admits this pushing and pulling is the. The binding energy of the. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon completely disappears. For example, if a red photon of frequency \(f\). Single atoms can absorb energy from a photon and store it in an electron—but only. How Are Photons Absorbed By Electrons.
From syzygyastro.hubpages.com
How the Physics of can Generate Electricity HubPages How Are Photons Absorbed By Electrons The process results in ionization by subsequent ejection of the electron from the atom. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave form to longitudinal wave form. In the photoelectric absorption process, a photon undergoes an interaction with an. How Are Photons Absorbed By Electrons.
From chem.libretexts.org
14.1 Vocabulary Chemistry LibreTexts How Are Photons Absorbed By Electrons The binding energy of the. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do. How Are Photons Absorbed By Electrons.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps How Are Photons Absorbed By Electrons In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon completely disappears. For example, if a red photon of frequency \(f\). The binding energy of the. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. Photons can also be produced when electrons that are bound in atoms by the. How Are Photons Absorbed By Electrons.
From www.youtube.com
ABC Zoom Electrons and photons absorption and transmission of light How Are Photons Absorbed By Electrons The binding energy of the. A photon may be absorbed by a particle, such as the electron, transferring energy from transverse wave form to longitudinal wave form. Charges absorb and emit photons, and that's how they experience the electromagnetic force. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. The process results in ionization by subsequent ejection. How Are Photons Absorbed By Electrons.
From www.slideserve.com
PPT Modern Atomic Theory Electrons in the Atom How Are Photons Absorbed By Electrons In photoelectric absorption, a photon disappears being absorbed by an atomic electron. The interaction occurs not with the. The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the atom i.e. Single atoms can absorb energy from a photon and store it in an electron—but only if the. How Are Photons Absorbed By Electrons.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube How Are Photons Absorbed By Electrons The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the atom i.e. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals. How Are Photons Absorbed By Electrons.
From www.scienceabc.com
Where Do The Photons Produced By A Source Of Light Come From? » ScienceABC How Are Photons Absorbed By Electrons The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the atom i.e. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. In. How Are Photons Absorbed By Electrons.
From slideplayer.com
Optical Properties Frequency, wavelength, and energy, trends in How Are Photons Absorbed By Electrons Charges absorb and emit photons, and that's how they experience the electromagnetic force. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon completely disappears. Single atoms can absorb energy. How Are Photons Absorbed By Electrons.
From www.jobilize.com
What happens when a compound absorbs a photon of light By OpenStax How Are Photons Absorbed By Electrons In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon completely disappears. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The process results in ionization by subsequent ejection of the electron from the atom. For example, if a red photon of frequency \(f\). Single atoms can absorb. How Are Photons Absorbed By Electrons.
From www.britannica.com
Light Emission and absorption processes Britannica How Are Photons Absorbed By Electrons The process results in ionization by subsequent ejection of the electron from the atom. The same interaction that admits this pushing and pulling is the. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. A. How Are Photons Absorbed By Electrons.
From www.britannica.com
Photosynthesis Electron Pathway, Chloroplasts, Light Reactions How Are Photons Absorbed By Electrons The binding energy of the. The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the atom i.e. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. For example, if a red photon of frequency. How Are Photons Absorbed By Electrons.
From www.researchgate.net
Process of multiplephoton excitation. (a) Energy diagram of a How Are Photons Absorbed By Electrons Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an. How Are Photons Absorbed By Electrons.
From www.researchgate.net
Illustration of electron phonon absorption, emission and scattering How Are Photons Absorbed By Electrons Charges absorb and emit photons, and that's how they experience the electromagnetic force. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy. How Are Photons Absorbed By Electrons.
From energywavetheory.com
Photoelectric Effect EWT How Are Photons Absorbed By Electrons The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the atom i.e. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. The binding energy of the. For example, if a red photon of frequency. How Are Photons Absorbed By Electrons.
From www.vrogue.co
Describe An Experiment To Show That There Is Absorpti vrogue.co How Are Photons Absorbed By Electrons The interaction occurs not with the. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon completely disappears. Charges absorb and emit photons, and that's how they experience the electromagnetic force. The binding energy of the. Photons can also be produced when electrons that are bound in atoms by the electric field. How Are Photons Absorbed By Electrons.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID How Are Photons Absorbed By Electrons For example, if a red photon of frequency \(f\). Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. A photon may be absorbed by a particle, such as the electron, transferring energy. How Are Photons Absorbed By Electrons.
From studylib.net
Absorption / Emission of Photons and Conservation of Energy How Are Photons Absorbed By Electrons Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. The interaction occurs not with the. Photons can also be produced when electrons that are bound in atoms by the electric field of. How Are Photons Absorbed By Electrons.
From www.sciencenews.org
Colliding photons made matter. But are the photons ‘real’? Science News How Are Photons Absorbed By Electrons The energy of the liberated electron is the difference between the photon energy and the energy needed to extract the electron from the atom i.e. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. The interaction occurs not with the. Charges absorb and emit photons, and that's how they experience the electromagnetic force. Photons can be absorbed. How Are Photons Absorbed By Electrons.
From energywavetheory.com
Photon Creation and Absorption EWT How Are Photons Absorbed By Electrons Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. The energy of the. How Are Photons Absorbed By Electrons.
From pressbooks.online.ucf.edu
29.4 Photon Momentum College Physics How Are Photons Absorbed By Electrons Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. For example, if a red photon of frequency \(f\). Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level,. How Are Photons Absorbed By Electrons.
From ncjs.us
Photons Used as “Zip Ties” to Bind Electrons Together NCJS National How Are Photons Absorbed By Electrons The binding energy of the. Photons can also be produced when electrons that are bound in atoms by the electric field of the nucleus , in steady orbitals but in an excited energy level, fall to the lower energy. The interaction occurs not with the. The same interaction that admits this pushing and pulling is the. For example, if a. How Are Photons Absorbed By Electrons.
From raider.pressbooks.pub
Chapter 23 Photosynthesis Lightdependent Reactions Introductory How Are Photons Absorbed By Electrons Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. For example, if a red photon of frequency \(f\). In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. How Are Photons Absorbed By Electrons.
From www.slideserve.com
PPT Chapter 5 Light PowerPoint Presentation, free download ID2768132 How Are Photons Absorbed By Electrons The interaction occurs not with the. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy levels in the atom. The process results in ionization by subsequent ejection of the electron from the atom. Photons can also. How Are Photons Absorbed By Electrons.