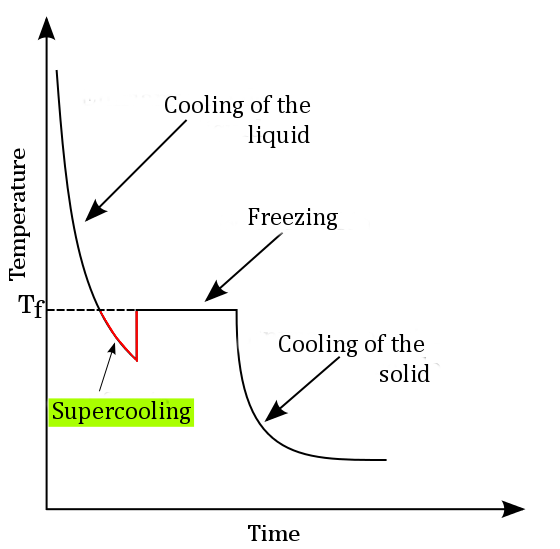
Super Cooling Stands For Cooling Of A Liquid . It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. Supercooling can happen because liquids are loose jumbles of atoms. — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. Pure water cooled to below 273k without freezing; supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid.
from www.vericormed.com
Supercooling can happen because liquids are loose jumbles of atoms. It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. Pure water cooled to below 273k without freezing; — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point.
Understanding Supercooling VeriCor Modifies Lab Freezer PCM Panel
Super Cooling Stands For Cooling Of A Liquid Supercooling can happen because liquids are loose jumbles of atoms. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. Pure water cooled to below 273k without freezing; Supercooling can happen because liquids are loose jumbles of atoms.
From hyte.com
Beginner's Guide to Custom Liquid Cooling HYTE Super Cooling Stands For Cooling Of A Liquid — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. Supercooling can happen because liquids are loose jumbles of atoms. It achieves this in the absence of a seed crystal or nucleus. Super Cooling Stands For Cooling Of A Liquid.
From www.trentonsystems.com
What is liquid cooling? Super Cooling Stands For Cooling Of A Liquid — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. Pure water cooled to below 273k without freezing; Supercooling can happen because. Super Cooling Stands For Cooling Of A Liquid.
From www.vericormed.com
Understanding Supercooling VeriCor Modifies Lab Freezer PCM Panel Super Cooling Stands For Cooling Of A Liquid — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. Supercooling can happen because liquids are loose jumbles of atoms. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. supercooling, also known as undercooling, is the process of lowering the. Super Cooling Stands For Cooling Of A Liquid.
From articles.informer.com
All you need to know about liquid cooling systems part I Super Cooling Stands For Cooling Of A Liquid Supercooling can happen because liquids are loose jumbles of atoms. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. It’s hard for such disordered atoms to lock. Super Cooling Stands For Cooling Of A Liquid.
From www.bitsandbytes-computers.com
Liquid Cooling and What You Need to Know! Super Cooling Stands For Cooling Of A Liquid supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: — supercooling, also known as. Super Cooling Stands For Cooling Of A Liquid.
From tas.com
Immersion Cooling Solutions 2 Phase Liquid Immersion Cooling Super Cooling Stands For Cooling Of A Liquid — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: Pure water cooled to below 273k without freezing; supercooling,. Super Cooling Stands For Cooling Of A Liquid.
From hometechfocus.com
Best Liquid Cooling Pc Kit Home Tech Super Cooling Stands For Cooling Of A Liquid supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic. Super Cooling Stands For Cooling Of A Liquid.
From www.cgdirector.com
Guide To AIOs (AllInOne) Liquid Coolers Super Cooling Stands For Cooling Of A Liquid — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. Supercooling can happen because liquids are loose jumbles of atoms. It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. — supercooling, also known as undercooling, is the process of lowering the temperature of. Super Cooling Stands For Cooling Of A Liquid.
From studybrewmaster.z21.web.core.windows.net
Why Does Supercooling Occur Super Cooling Stands For Cooling Of A Liquid — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it. Super Cooling Stands For Cooling Of A Liquid.
From dcx.eu
DCX The Liquid Cooling Company DCX The Liquid Cooling Company Super Cooling Stands For Cooling Of A Liquid — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. Pure water cooled to below 273k without freezing; It’s hard for such disordered atoms to lock themselves into the crystal structure of. Super Cooling Stands For Cooling Of A Liquid.
From www.electronics-cooling.com
Liquid Cooling Options for HighPerformance Computing Centers Super Cooling Stands For Cooling Of A Liquid Supercooling can happen because liquids are loose jumbles of atoms. It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: Pure water cooled to below 273k without freezing; — supercooling, also known as undercooling, is the process of. Super Cooling Stands For Cooling Of A Liquid.
From atelier-yuwa.ciao.jp
All You Need To Know About Liquid Cooling Systems Part II atelier Super Cooling Stands For Cooling Of A Liquid Pure water cooled to below 273k without freezing; It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. — a pure liquid, therefore, needs. Super Cooling Stands For Cooling Of A Liquid.
From www.miniphysics.com
Unveiling The Magic Of Supercooling How To Instantly Freeze Water Super Cooling Stands For Cooling Of A Liquid It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. Supercooling can happen because liquids are loose jumbles of atoms. — when a liquid is supercooled its structure doesn't change. Super Cooling Stands For Cooling Of A Liquid.
From www.supermicro.com
Liquid Cooled Servers & Water Cooled Rack Servers Supermicro Super Cooling Stands For Cooling Of A Liquid — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it. Super Cooling Stands For Cooling Of A Liquid.
From www.cgdirector.com
Guide To AIOs (AllInOne) Liquid Coolers Super Cooling Stands For Cooling Of A Liquid — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. Supercooling can happen because liquids are loose jumbles of atoms. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. It achieves. Super Cooling Stands For Cooling Of A Liquid.
From www.cescorp.ca
Immersion Cooling vs Direct Liquid Cooling in Advanced Data Centres Super Cooling Stands For Cooling Of A Liquid Pure water cooled to below 273k without freezing; — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. — supercooling, also known as undercooling, is the process. Super Cooling Stands For Cooling Of A Liquid.
From www.custompcguide.net
Gallery of an Awesome Wallmounted Custom PC with Beautiful Liquid Super Cooling Stands For Cooling Of A Liquid — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. It achieves this in the absence of a. Super Cooling Stands For Cooling Of A Liquid.
From justyoumarket.com
Best Liquid Cooling System Kit Home Future Market Super Cooling Stands For Cooling Of A Liquid — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without. Super Cooling Stands For Cooling Of A Liquid.
From www.eurekalert.org
Liquid cooling moves onto the chip for denser EurekAlert! Super Cooling Stands For Cooling Of A Liquid — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. Supercooling can happen because liquids are loose jumbles. Super Cooling Stands For Cooling Of A Liquid.
From liquidcoolingnadairi.blogspot.com
Liquid Cooling Liquid Cooling Loop Super Cooling Stands For Cooling Of A Liquid — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: Supercooling can happen because liquids are loose jumbles of atoms. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid. Super Cooling Stands For Cooling Of A Liquid.
From dailyspect.com
The 10 Best Liquid Cooling 80Mm Your Home Life Super Cooling Stands For Cooling Of A Liquid Supercooling can happen because liquids are loose jumbles of atoms. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. Pure water cooled to below 273k without freezing;. Super Cooling Stands For Cooling Of A Liquid.
From www.tech-wonders.com
How Liquid Cooling Works, Types of Liquid Cooling Systems, and How to Super Cooling Stands For Cooling Of A Liquid It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: It’s hard for. Super Cooling Stands For Cooling Of A Liquid.
From www.youtube.com
How to Build Liquid Cooling System for PC at Home YouTube Super Cooling Stands For Cooling Of A Liquid supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. Pure water cooled to below 273k without freezing; It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. It’s hard for such disordered atoms to lock themselves into. Super Cooling Stands For Cooling Of A Liquid.
From pinocollection.com
Which Is The Best Cooler Master Seidon 120Xl Liquid Cpu Water Cooling Super Cooling Stands For Cooling Of A Liquid Supercooling can happen because liquids are loose jumbles of atoms. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. Pure water cooled to below 273k without freezing;. Super Cooling Stands For Cooling Of A Liquid.
From www.interactive.com.au
Liquid Immersion Cooling Unleashing NextGen Innovations Super Cooling Stands For Cooling Of A Liquid Supercooling can happen because liquids are loose jumbles of atoms. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. — a pure liquid, therefore, needs to be significantly supercooled before. Super Cooling Stands For Cooling Of A Liquid.
From www.akcp.com
Liquid Immersion Cooling A Journey to Better Cooling Super Cooling Stands For Cooling Of A Liquid Supercooling can happen because liquids are loose jumbles of atoms. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming. Super Cooling Stands For Cooling Of A Liquid.
From www.boxtechy.com
Immersion Cooling The New Horizon of Liquid Cooling BoxTechy Super Cooling Stands For Cooling Of A Liquid supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. — a pure liquid, therefore, needs to. Super Cooling Stands For Cooling Of A Liquid.
From dcx.eu
10 important considerations on implementation of liquid cooling systems Super Cooling Stands For Cooling Of A Liquid Pure water cooled to below 273k without freezing; — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a. Super Cooling Stands For Cooling Of A Liquid.
From onceownedby.com
The 10 Best Nzxt Water Liquid Cooling Home Gadgets Super Cooling Stands For Cooling Of A Liquid — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. Pure water cooled to below 273k without freezing; — supercooling, also known as undercooling, is the process. Super Cooling Stands For Cooling Of A Liquid.
From submer.com
What Is Immersion Cooling? Liquid Immersion Cooling Submer Super Cooling Stands For Cooling Of A Liquid It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. — a pure liquid, therefore,. Super Cooling Stands For Cooling Of A Liquid.
From thetechmirror.com
What Is Liquid Cooling Technology And How Does It Work? Super Cooling Stands For Cooling Of A Liquid Supercooling can happen because liquids are loose jumbles of atoms. It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. Pure water cooled to below 273k without freezing; — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: — supercooling, also known as undercooling, is the process of. Super Cooling Stands For Cooling Of A Liquid.
From www.cstheatexchanger.com
What is Immersion Liquid Cooling? Changzhou Vrcoolertech Super Cooling Stands For Cooling Of A Liquid Supercooling can happen because liquids are loose jumbles of atoms. It’s hard for such disordered atoms to lock themselves into the crystal structure of a solid. — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. Pure water cooled to below 273k without freezing; — supercooling, also known as undercooling,. Super Cooling Stands For Cooling Of A Liquid.
From www.researchgate.net
Concept of supercooling preservation controlled by stepwise cooling Super Cooling Stands For Cooling Of A Liquid It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. Supercooling can happen because liquids are loose jumbles of atoms. — a pure liquid, therefore, needs to. Super Cooling Stands For Cooling Of A Liquid.
From www.powerelectronictips.com
Liquid cooling for highperformance thermal management Power Super Cooling Stands For Cooling Of A Liquid Pure water cooled to below 273k without freezing; — supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid. — a pure liquid, therefore, needs to be significantly supercooled before homogeneous nucleation occurs: It’s hard for such disordered atoms to lock. Super Cooling Stands For Cooling Of A Liquid.
From www.supermicro.com
RackScale Liquid Cooling Solutions Supermicro Super Cooling Stands For Cooling Of A Liquid — when a liquid is supercooled its structure doesn't change significantly, only the particles have less kinetic energy. Supercooling can happen because liquids are loose jumbles of atoms. supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point. It’s hard for such disordered atoms to lock. Super Cooling Stands For Cooling Of A Liquid.