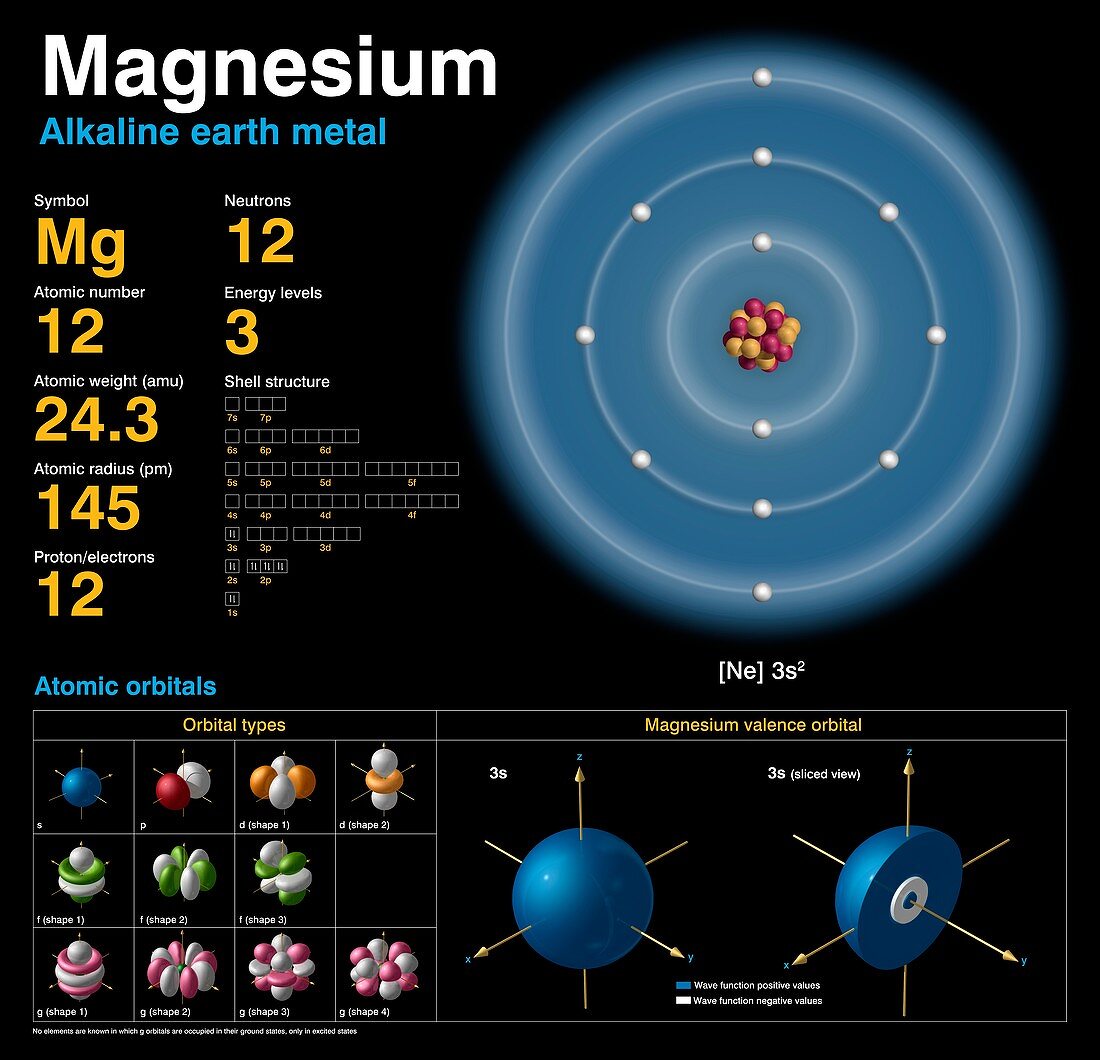
Atomic Mass Of Magnesium Isotopes . Mass numbers of typical isotopes of magnesium. This is approximately the sum of the number of protons and neutrons in the nucleus. 24 mg, 25 mg, and 26 mg. This table shows information about naturally occuring. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: There are 19 radioisotopes that have been.
from mavink.com
46 rows magnesium (12 mg) naturally occurs in three stable isotopes: There are 19 radioisotopes that have been. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. This table shows information about naturally occuring. 24 mg, 25 mg, and 26 mg. This is approximately the sum of the number of protons and neutrons in the nucleus. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. Mass numbers of typical isotopes of magnesium.
Atomic Structure Of Magnesium
Atomic Mass Of Magnesium Isotopes 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. 24 mg, 25 mg, and 26 mg. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. There are 19 radioisotopes that have been. Mass numbers of typical isotopes of magnesium. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: This table shows information about naturally occuring. This is approximately the sum of the number of protons and neutrons in the nucleus.
From www.slideserve.com
PPT Isotopes of Magnesium PowerPoint Presentation ID4271266 Atomic Mass Of Magnesium Isotopes These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. Mass numbers of typical isotopes of magnesium. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. This is. Atomic Mass Of Magnesium Isotopes.
From www.britannica.com
magnesium Description, Properties, & Compounds Britannica Atomic Mass Of Magnesium Isotopes For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. This table shows information about naturally occuring. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: These values tell you that a magnesium atom has twice the mass of a carbon atom,. Atomic Mass Of Magnesium Isotopes.
From www.thesciencehive.co.uk
Atomic Structure and Isotopes* — the science sauce Atomic Mass Of Magnesium Isotopes This table shows information about naturally occuring. Mass numbers of typical isotopes of magnesium. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. 24 mg, 25 mg, and 26 mg. These values tell you that a magnesium atom has twice the mass of. Atomic Mass Of Magnesium Isotopes.
From www.alamy.com
magnesium isotopes atomic structure backdrop physics theory Atomic Mass Of Magnesium Isotopes 24 mg, 25 mg, and 26 mg. There are 19 radioisotopes that have been. This is approximately the sum of the number of protons and neutrons in the nucleus. Mass numbers of typical isotopes of magnesium. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25,. Atomic Mass Of Magnesium Isotopes.
From www.slideserve.com
PPT Isotopes PowerPoint Presentation, free download ID3340712 Atomic Mass Of Magnesium Isotopes Mass numbers of typical isotopes of magnesium. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. This table. Atomic Mass Of Magnesium Isotopes.
From www.alamy.com
magnesium isotopes atomic structure backdrop physics theory Atomic Mass Of Magnesium Isotopes For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. 24 mg, 25 mg, and 26 mg. There are 19 radioisotopes that have been. This is approximately the sum of the number of protons and neutrons in the nucleus. This table shows information about. Atomic Mass Of Magnesium Isotopes.
From www.slideserve.com
PPT Isotopes of Magnesium PowerPoint Presentation, free download ID Atomic Mass Of Magnesium Isotopes For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. There are 19 radioisotopes that have been. Mass numbers of typical isotopes of magnesium. 24 mg, 25 mg, and 26 mg. This is approximately the sum of the number of protons and neutrons in. Atomic Mass Of Magnesium Isotopes.
From www.alamy.com
magnesium isotopes atomic structure backdrop physics theory Atomic Mass Of Magnesium Isotopes 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. 24 mg, 25 mg, and 26 mg. These values tell you that a magnesium atom has twice the mass of a carbon atom,. Atomic Mass Of Magnesium Isotopes.
From www.dreamstime.com
Illustration of Magnesium Isotopes Atomic Structure Stock Illustration Atomic Mass Of Magnesium Isotopes These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. Mass numbers of typical isotopes of magnesium. There are 19 radioisotopes that have been. This table shows information about naturally occuring. For example, magnesium exists as a mixture of three isotopes, each with an atomic. Atomic Mass Of Magnesium Isotopes.
From slideplayer.com
Atomic Mass Na The atomic mass of an element ppt download Atomic Mass Of Magnesium Isotopes This table shows information about naturally occuring. 24 mg, 25 mg, and 26 mg. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. Mass numbers of typical isotopes of magnesium. This is approximately the sum of the number of protons and neutrons in. Atomic Mass Of Magnesium Isotopes.
From www.numerade.com
SOLVEDThere are three naturally occurring isotopes of the element Atomic Mass Of Magnesium Isotopes For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. There are 19 radioisotopes that have been. This table shows information about naturally occuring. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more. Atomic Mass Of Magnesium Isotopes.
From www.alamy.com
magnesium isotopes atomic structure backdrop physics theory Atomic Mass Of Magnesium Isotopes This table shows information about naturally occuring. 24 mg, 25 mg, and 26 mg. This is approximately the sum of the number of protons and neutrons in the nucleus. There are 19 radioisotopes that have been. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: Mass numbers of typical isotopes of magnesium. For example, magnesium exists as a. Atomic Mass Of Magnesium Isotopes.
From www.livescience.com
Scientists create neverbeforeseen isotope of magnesium Live Science Atomic Mass Of Magnesium Isotopes This table shows information about naturally occuring. This is approximately the sum of the number of protons and neutrons in the nucleus. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. There are 19 radioisotopes that have been. 24 mg, 25 mg, and 26. Atomic Mass Of Magnesium Isotopes.
From mavink.com
Atomic Structure Of Magnesium Atomic Mass Of Magnesium Isotopes There are 19 radioisotopes that have been. Mass numbers of typical isotopes of magnesium. This is approximately the sum of the number of protons and neutrons in the nucleus. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. 24 mg, 25 mg, and. Atomic Mass Of Magnesium Isotopes.
From www.researchgate.net
values for the atomic masses of the magnesium isotopes, the constants Atomic Mass Of Magnesium Isotopes This table shows information about naturally occuring. This is approximately the sum of the number of protons and neutrons in the nucleus. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. For example, magnesium exists as a mixture of three isotopes, each with an. Atomic Mass Of Magnesium Isotopes.
From slideplayer.com
Chapter 4 Atoms and Elements ppt download Atomic Mass Of Magnesium Isotopes There are 19 radioisotopes that have been. This table shows information about naturally occuring. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more. Atomic Mass Of Magnesium Isotopes.
From www.slideserve.com
PPT Atoms, Ions and Isotopes PowerPoint Presentation ID5401475 Atomic Mass Of Magnesium Isotopes 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. This is approximately the sum of the number of protons and neutrons in the nucleus. There are 19 radioisotopes that have been. 24 mg,. Atomic Mass Of Magnesium Isotopes.
From www.alamy.com
A schematic illustration of magnesium isotopes atomic structure Atomic Mass Of Magnesium Isotopes There are 19 radioisotopes that have been. This is approximately the sum of the number of protons and neutrons in the nucleus. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. 24 mg,. Atomic Mass Of Magnesium Isotopes.
From slidetodoc.com
Atomic Mass and Isotope Objectives SWBAT calculate the Atomic Mass Of Magnesium Isotopes 24 mg, 25 mg, and 26 mg. This is approximately the sum of the number of protons and neutrons in the nucleus. Mass numbers of typical isotopes of magnesium. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. There are 19 radioisotopes that. Atomic Mass Of Magnesium Isotopes.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Atomic Mass Of Magnesium Isotopes These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. Mass numbers of typical isotopes of magnesium. This table shows information about naturally occuring. There are 19 radioisotopes that have been. 24 mg, 25 mg, and 26 mg. 46 rows magnesium (12 mg) naturally occurs. Atomic Mass Of Magnesium Isotopes.
From www.slideserve.com
PPT Isotopes and Atomic Mass PowerPoint Presentation, free download Atomic Mass Of Magnesium Isotopes Mass numbers of typical isotopes of magnesium. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. This table. Atomic Mass Of Magnesium Isotopes.
From present5.com
Isotopes Atoms of the same element with Atomic Mass Of Magnesium Isotopes These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. This is approximately the sum of the number of. Atomic Mass Of Magnesium Isotopes.
From noradesnhharper.blogspot.com
Relative Atomic Mass of Magnesium Atomic Mass Of Magnesium Isotopes This table shows information about naturally occuring. There are 19 radioisotopes that have been. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: This is approximately the sum of the number of protons. Atomic Mass Of Magnesium Isotopes.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Atomic Mass Of Magnesium Isotopes 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: There are 19 radioisotopes that have been. This table shows information about naturally occuring. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. These values tell you that a magnesium atom has. Atomic Mass Of Magnesium Isotopes.
From periodictableguide.com
Magnesium (Mg) Periodic Table (Element Information & More) Atomic Mass Of Magnesium Isotopes Mass numbers of typical isotopes of magnesium. This is approximately the sum of the number of protons and neutrons in the nucleus. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. This. Atomic Mass Of Magnesium Isotopes.
From www.sliderbase.com
Atomic Mass Magnesium has three isotopes. 78.99 magnesium 24 with a Atomic Mass Of Magnesium Isotopes These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: Mass numbers of typical isotopes of magnesium. 24 mg, 25 mg, and 26 mg. This is approximately the sum of the number of protons. Atomic Mass Of Magnesium Isotopes.
From www.dreamstime.com
Illustration of Magnesium Isotopes Atomic Structure Stock Illustration Atomic Mass Of Magnesium Isotopes There are 19 radioisotopes that have been. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. This is approximately the sum of the number of protons and neutrons in the nucleus. For example, magnesium exists as a mixture of three isotopes, each with an. Atomic Mass Of Magnesium Isotopes.
From www.slideserve.com
PPT Isotopes and Atomic Mass PowerPoint Presentation, free download Atomic Mass Of Magnesium Isotopes For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. This is approximately the sum of the number of protons and neutrons in the nucleus. Mass numbers of typical isotopes of magnesium. These values tell you that a magnesium atom has twice the mass. Atomic Mass Of Magnesium Isotopes.
From www.youtube.com
How to Find the Mass of One Atom of Magnesium (Mg) YouTube Atomic Mass Of Magnesium Isotopes Mass numbers of typical isotopes of magnesium. This is approximately the sum of the number of protons and neutrons in the nucleus. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. 24 mg, 25 mg, and 26 mg. These values tell you that. Atomic Mass Of Magnesium Isotopes.
From www.chegg.com
Solved Calculate the elemental atomic mass of Mg if the Atomic Mass Of Magnesium Isotopes For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. This is approximately the sum of the number of protons and neutrons in the nucleus. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times. Atomic Mass Of Magnesium Isotopes.
From www.alamy.com
magnesium isotopes atomic structure backdrop physics theory Atomic Mass Of Magnesium Isotopes Mass numbers of typical isotopes of magnesium. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. This table shows information about naturally occuring. 24 mg, 25 mg, and 26 mg. There are 19 radioisotopes that have been. 46 rows magnesium (12 mg) naturally occurs. Atomic Mass Of Magnesium Isotopes.
From www.slideserve.com
PPT The Atom Atomic Number Mass Number Isotopes PowerPoint Atomic Mass Of Magnesium Isotopes For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. Mass numbers of typical isotopes of magnesium. These values tell you that a magnesium atom has twice the mass of a carbon atom, and 24 times more mass than a hydrogen atom. There are. Atomic Mass Of Magnesium Isotopes.
From www.nuclear-power.com
Magnesium Atomic Number Atomic Mass Density of Magnesium Atomic Mass Of Magnesium Isotopes This is approximately the sum of the number of protons and neutrons in the nucleus. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: This table shows information about naturally occuring. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. Mass. Atomic Mass Of Magnesium Isotopes.
From www.alamy.com
magnesium isotopes atomic structure backdrop physics theory Atomic Mass Of Magnesium Isotopes This is approximately the sum of the number of protons and neutrons in the nucleus. 24 mg, 25 mg, and 26 mg. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: There are 19 radioisotopes that have been. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass. Atomic Mass Of Magnesium Isotopes.
From wisc.pb.unizin.org
Isotopes, Atomic Mass, and Mass Spectrometry (M2Q3) UWMadison Atomic Mass Of Magnesium Isotopes This is approximately the sum of the number of protons and neutrons in the nucleus. For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26,. There are 19 radioisotopes that have been. 24 mg, 25 mg, and 26 mg. Mass numbers of typical isotopes. Atomic Mass Of Magnesium Isotopes.