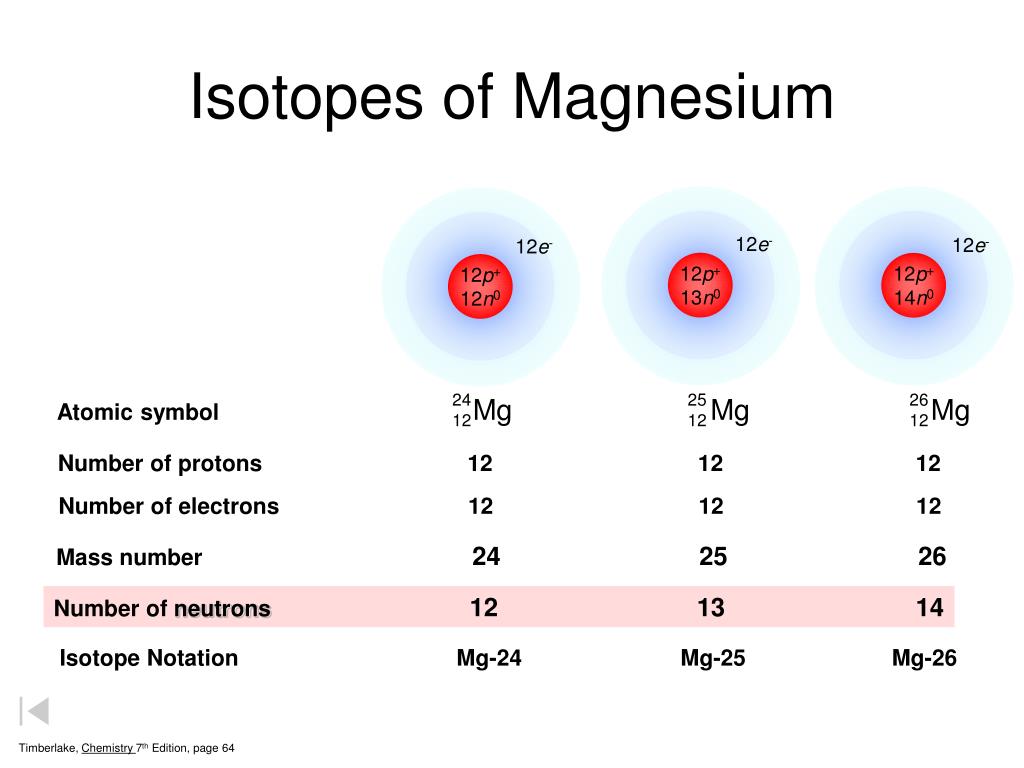
Magnesium Neutrons Electrons . Protons et neutrons dans le magnésium. Electron configuration of magnesium is [ne] 3s2. #z#, the atomic number of #mg# is #12#. 3 (nombre d'électrons ou de protons) nombre de masse : 6 (nombre de nucléons = nb de protons. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Possible oxidation states are +2. #z# refers to the number of (nuclear) protons. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. It has an atomic weight of 24.305 and a.
from www.slideserve.com
Determine the number of protons and electrons in an atom. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Protons et neutrons dans le magnésium. 6 (nombre de nucléons = nb de protons. 3 (nombre d'électrons ou de protons) nombre de masse : Electron configuration of magnesium is [ne] 3s2. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Possible oxidation states are +2. It has an atomic weight of 24.305 and a.
PPT Isotopes of Magnesium PowerPoint Presentation, free download ID
Magnesium Neutrons Electrons Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Describe the locations, charges, and masses of the three main subatomic particles. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. 6 (nombre de nucléons = nb de protons. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Determine the number of protons and electrons in an atom. #z# refers to the number of (nuclear) protons. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Electron configuration of magnesium is [ne] 3s2. It has an atomic weight of 24.305 and a. Protons et neutrons dans le magnésium. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. Possible oxidation states are +2. 3 (nombre d'électrons ou de protons) nombre de masse : #z#, the atomic number of #mg# is #12#.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Neutrons Electrons Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. #z#, the atomic number of #mg# is #12#. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Understanding the lewis structure of magnesium provides insights into its bonding. Magnesium Neutrons Electrons.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Neutrons Electrons Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Describe the locations, charges,. Magnesium Neutrons Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Magnesium Neutrons Electrons Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Possible oxidation states are +2. It has an atomic weight of 24.305 and a. #z#, the atomic number of #mg# is #12#. Protons et neutrons dans le magnésium. Determine the number of protons and electrons in an atom. 3 (nombre. Magnesium Neutrons Electrons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Neutrons Electrons #z# refers to the number of (nuclear) protons. Electron configuration of magnesium is [ne] 3s2. Determine the number of protons and electrons in an atom. 3 (nombre d'électrons ou de protons) nombre de masse : Protons et neutrons dans le magnésium. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole. Magnesium Neutrons Electrons.
From www.dreamstime.com
Magnesium Atom, with Mass and Energy Levels. Stock Vector Magnesium Neutrons Electrons 6 (nombre de nucléons = nb de protons. Determine the number of protons and electrons in an atom. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. 3 (nombre d'électrons ou de protons) nombre de masse : Describe the locations, charges, and masses of the three main subatomic particles.. Magnesium Neutrons Electrons.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Neutrons Electrons Protons et neutrons dans le magnésium. 3 (nombre d'électrons ou de protons) nombre de masse : #z# refers to the number of (nuclear) protons. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. It has an atomic weight of 24.305 and a. #z#, the atomic number of #mg# is #12#. Electron configuration of magnesium. Magnesium Neutrons Electrons.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Neutrons Electrons Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. It has an atomic weight of 24.305 and a. Possible oxidation states are +2. 6 (nombre de nucléons = nb de protons. Electron configuration of magnesium is [ne] 3s2. Determine the number of protons and electrons in an atom.. Magnesium Neutrons Electrons.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Neutrons Electrons #z#, the atomic number of #mg# is #12#. 3 (nombre d'électrons ou de protons) nombre de masse : Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. Electron configuration of magnesium is [ne] 3s2. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular. Magnesium Neutrons Electrons.
From sciencenotes.org
Magnesium Facts Magnesium Neutrons Electrons Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. #z# refers to the number of (nuclear) protons. #z#, the atomic number of #mg# is #12#. 3 (nombre d'électrons ou de protons) nombre de. Magnesium Neutrons Electrons.
From valenceelectrons.com
How Many Protons,Neutrons and Electrons Does Magnesium Have? Magnesium Neutrons Electrons Determine the number of protons and electrons in an atom. It has an atomic weight of 24.305 and a. Possible oxidation states are +2. Describe the locations, charges, and masses of the three main subatomic particles. Protons et neutrons dans le magnésium. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number. Magnesium Neutrons Electrons.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Neutrons Electrons #z# refers to the number of (nuclear) protons. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. #z#, the atomic number of #mg# is #12#. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Electron configuration of. Magnesium Neutrons Electrons.
From ar.inspiredpencil.com
Magnesium Atom 3d Model Magnesium Neutrons Electrons 3 (nombre d'électrons ou de protons) nombre de masse : Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Describe the locations, charges, and masses of the three main subatomic particles. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Le magnésium est. Magnesium Neutrons Electrons.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium Neutrons Electrons 6 (nombre de nucléons = nb de protons. Protons et neutrons dans le magnésium. Possible oxidation states are +2. #z#, the atomic number of #mg# is #12#. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. #z# refers to the number of (nuclear) protons. Describe the locations, charges, and masses of the three main. Magnesium Neutrons Electrons.
From www.benjamin-mills.com
Electron arrangements Magnesium Neutrons Electrons It has an atomic weight of 24.305 and a. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Describe the locations, charges, and masses of the three main subatomic particles. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. Possible oxidation states. Magnesium Neutrons Electrons.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Neutrons Electrons Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. It has an atomic weight of 24.305 and a. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Magnesium is the 12th element in the periodic table and has a symbol of mg. Magnesium Neutrons Electrons.
From material-properties.org
Magnesium Protons Neutrons Electrons Electron Configuration Magnesium Neutrons Electrons 3 (nombre d'électrons ou de protons) nombre de masse : Electron configuration of magnesium is [ne] 3s2. #z#, the atomic number of #mg# is #12#. It has an atomic weight of 24.305 and a. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Protons et neutrons dans le magnésium.. Magnesium Neutrons Electrons.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C013/1519 Science Photo Library Magnesium Neutrons Electrons Protons et neutrons dans le magnésium. Determine the number of protons and electrons in an atom. Possible oxidation states are +2. Electron configuration of magnesium is [ne] 3s2. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. 3 (nombre d'électrons ou de protons) nombre de masse : Le nombre total de protons dans le. Magnesium Neutrons Electrons.
From www.dreamstime.com
Atom Of Magnesium With Detailed Core And Its 12 Electrons Stock Magnesium Neutrons Electrons Possible oxidation states are +2. It has an atomic weight of 24.305 and a. 6 (nombre de nucléons = nb de protons. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number. Magnesium Neutrons Electrons.
From www.slideserve.com
PPT Part A Atomic Structure PowerPoint Presentation, free download Magnesium Neutrons Electrons Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Possible oxidation states are +2. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. #z# refers to the number of (nuclear) protons. 6 (nombre de nucléons = nb de protons. Electron configuration of. Magnesium Neutrons Electrons.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Neutrons Electrons Protons et neutrons dans le magnésium. #z# refers to the number of (nuclear) protons. It has an atomic weight of 24.305 and a. Possible oxidation states are +2. Describe the locations, charges, and masses of the three main subatomic particles. Electron configuration of magnesium is [ne] 3s2. 3 (nombre d'électrons ou de protons) nombre de masse : Understanding the lewis. Magnesium Neutrons Electrons.
From stock.adobe.com
Magnesium element with symbol Mg and atomic number 12.isolated Magnesium Neutrons Electrons Protons et neutrons dans le magnésium. Electron configuration of magnesium is [ne] 3s2. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. 3 (nombre d'électrons ou de protons). Magnesium Neutrons Electrons.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Magnesium Neutrons Electrons Determine the number of protons and electrons in an atom. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. #z#, the atomic number of #mg# is #12#. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Le magnésium est un élément chimique de. Magnesium Neutrons Electrons.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Magnesium Neutrons Electrons #z# refers to the number of (nuclear) protons. Describe the locations, charges, and masses of the three main subatomic particles. Electron configuration of magnesium is [ne] 3s2. Protons et neutrons dans le magnésium. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Le nombre total de protons dans le. Magnesium Neutrons Electrons.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Magnesium Neutrons Electrons Possible oxidation states are +2. #z#, the atomic number of #mg# is #12#. Describe the locations, charges, and masses of the three main subatomic particles. It has an atomic weight of 24.305 and a. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. Determine the number of protons. Magnesium Neutrons Electrons.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Neutrons Electrons Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. It has an atomic weight of 24.305 and a. 6 (nombre de nucléons = nb de protons. #z# refers to the number of (nuclear) protons. #z#, the atomic number of #mg# is #12#. Electron configuration of magnesium is [ne]. Magnesium Neutrons Electrons.
From nursehub.com
Electron Shells NurseHub Magnesium Neutrons Electrons Determine the number of protons and electrons in an atom. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Possible oxidation states are +2. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Protons et neutrons dans le. Magnesium Neutrons Electrons.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Neutrons Electrons Possible oxidation states are +2. It has an atomic weight of 24.305 and a. Describe the locations, charges, and masses of the three main subatomic particles. Protons et neutrons dans le magnésium. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie qu’il y a 12 protons dans son noyau. Understanding the lewis structure of magnesium provides. Magnesium Neutrons Electrons.
From www.slideserve.com
PPT Isotopes of Magnesium PowerPoint Presentation, free download ID Magnesium Neutrons Electrons It has an atomic weight of 24.305 and a. #z#, the atomic number of #mg# is #12#. #z# refers to the number of (nuclear) protons. Determine the number of protons and electrons in an atom. 6 (nombre de nucléons = nb de protons. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Magnesium is. Magnesium Neutrons Electrons.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Neutrons Electrons #z#, the atomic number of #mg# is #12#. Electron configuration of magnesium is [ne] 3s2. 6 (nombre de nucléons = nb de protons. 3 (nombre d'électrons ou de protons) nombre de masse : Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Possible oxidation states are +2. It has. Magnesium Neutrons Electrons.
From www.shutterstock.com
Magnesium Periodic Table Element Atomic Model Stock Vector (Royalty Magnesium Neutrons Electrons It has an atomic weight of 24.305 and a. 6 (nombre de nucléons = nb de protons. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Determine the number of protons and electrons in an atom. Le magnésium est un élément chimique de numéro atomique 12, ce qui signifie. Magnesium Neutrons Electrons.
From www.alamy.com
Atom Symbol for Magnesium Stock Vector Image & Art Alamy Magnesium Neutrons Electrons Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Determine the number of protons and electrons in an atom. Le magnésium est un élément chimique de numéro atomique 12,. Magnesium Neutrons Electrons.
From www.youtube.com
Electronic configuration for Magnesium (Mg) spdf Trick Chemistry Magnesium Neutrons Electrons Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. #z# refers to the number of (nuclear) protons. Describe the locations, charges, and masses of the three main subatomic particles. 6 (nombre de nucléons =. Magnesium Neutrons Electrons.
From periodictableguide.com
Magnesium (Mg) Periodic Table (Element Information & More) Magnesium Neutrons Electrons Determine the number of protons and electrons in an atom. Electron configuration of magnesium is [ne] 3s2. It has an atomic weight of 24.305 and a. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole z. Magnesium is the 12th element in the periodic table and has a symbol of. Magnesium Neutrons Electrons.
From www.slideserve.com
PPT Nuclear model of atom PowerPoint Presentation, free download ID Magnesium Neutrons Electrons It has an atomic weight of 24.305 and a. Determine the number of protons and electrons in an atom. Magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. #z#, the atomic number of #mg# is #12#. Electron configuration of magnesium is [ne] 3s2. #z# refers to the number of. Magnesium Neutrons Electrons.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Neutrons Electrons #z#, the atomic number of #mg# is #12#. Understanding the lewis structure of magnesium provides insights into its bonding behavior and molecular interactions. Describe the locations, charges, and masses of the three main subatomic particles. Protons et neutrons dans le magnésium. Possible oxidation states are +2. It has an atomic weight of 24.305 and a. Electron configuration of magnesium is. Magnesium Neutrons Electrons.