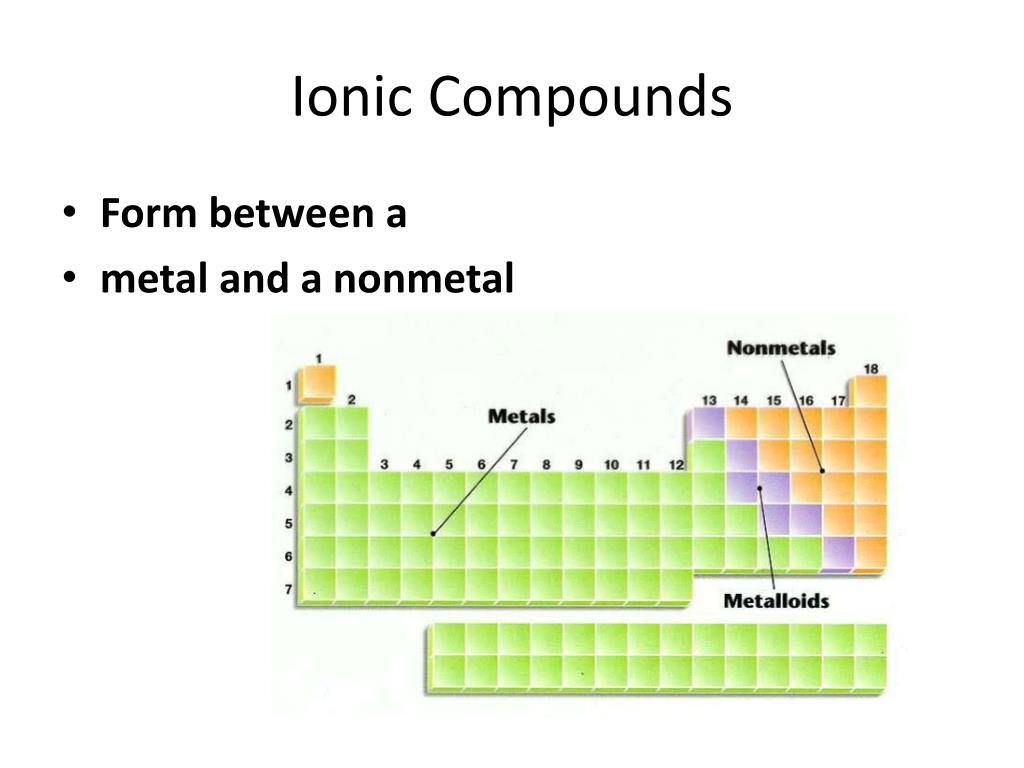
Are Ionic Compounds Metal . Binary ionic compounds are composed of just two elements: Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Such a bond forms when the valence. The formation of ionic compounds. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. A metal (which forms the cations) and a nonmetal. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Covalent bonds are highly stable bonds with. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. See the study guide on the.
from www.slideserve.com
Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence. A metal (which forms the cations) and a nonmetal. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Covalent bonds are highly stable bonds with. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Binary ionic compounds are composed of just two elements: Ionic compounds tend to be crystalline structures with high melting points that are water soluble.
PPT Ion Charge and the Formulas of Ionic Compounds PowerPoint
Are Ionic Compounds Metal Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. See the study guide on the. The formation of ionic compounds. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Covalent bonds are highly stable bonds with. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Such a bond forms when the valence. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. A metal (which forms the cations) and a nonmetal. Binary ionic compounds are composed of just two elements: Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron.
From sciencenotes.org
Naming Ionic Compounds Nomenclature Rules Are Ionic Compounds Metal Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. See the study guide on the. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. In covalent compounds, electrons. Are Ionic Compounds Metal.
From courses.lumenlearning.com
Molecular and Ionic Compounds Chemistry for Majors Are Ionic Compounds Metal In covalent compounds, electrons are shared between bonded atoms and are simultaneously. A metal (which forms the cations) and a nonmetal. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. See the study guide on the. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions,. Are Ionic Compounds Metal.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Are Ionic Compounds Metal A metal (which forms the cations) and a nonmetal. Binary ionic compounds are composed of just two elements: Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions,. Are Ionic Compounds Metal.
From www.animalia-life.club
Ionic Compounds Periodic Table Are Ionic Compounds Metal The formation of ionic compounds. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Covalent bonds are highly stable bonds with. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Such. Are Ionic Compounds Metal.
From www.slideserve.com
PPT NAMING IONIC COMPOUNDS PowerPoint Presentation, free download Are Ionic Compounds Metal Ionic compounds tend to be crystalline structures with high melting points that are water soluble. See the study guide on the. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Such a bond. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Science 10 Chapter 4.2 Names and Formulas of Ionic Compounds Are Ionic Compounds Metal Such a bond forms when the valence. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. A metal (which forms the cations) and a nonmetal. See the study guide on the. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Binary ionic compounds. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free Are Ionic Compounds Metal The formation of ionic compounds. See the study guide on the. Binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Ionic compounds contain both cations and anions in a ratio that results in no net electrical. Are Ionic Compounds Metal.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Are Ionic Compounds Metal See the study guide on the. Covalent bonds are highly stable bonds with. Such a bond forms when the valence. A metal (which forms the cations) and a nonmetal. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Binary ionic compounds are composed of just two elements: Ionic bond, type of linkage formed from. Are Ionic Compounds Metal.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Are Ionic Compounds Metal Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. Such a bond forms when the valence. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Covalent bonds are highly stable bonds. Are Ionic Compounds Metal.
From rachaelmeowgrimes.blogspot.com
Do Calcium and Rubidium Form an Ionic Compound Are Ionic Compounds Metal Binary ionic compounds are composed of just two elements: Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature.. Are Ionic Compounds Metal.
From www.nagwa.com
Question Video Determining Which Two Types of Elements Form an Ionic Are Ionic Compounds Metal In covalent compounds, electrons are shared between bonded atoms and are simultaneously. See the study guide on the. The formation of ionic compounds. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Such a bond forms when the valence. Ionic compounds tend to be crystalline structures with high melting points that are water soluble.. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID Are Ionic Compounds Metal See the study guide on the. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Such a bond forms when. Are Ionic Compounds Metal.
From studylib.net
Ionic Compounds and Metals Are Ionic Compounds Metal A metal (which forms the cations) and a nonmetal. Such a bond forms when the valence. Covalent bonds are highly stable bonds with. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Binary ionic compounds are composed of just two elements: Ionic compounds contain both cations and anions in a ratio that results in. Are Ionic Compounds Metal.
From www.animalia-life.club
Ionic Compounds Periodic Table Are Ionic Compounds Metal A metal (which forms the cations) and a nonmetal. See the study guide on the. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Ionic compounds have high melting and boiling points, so they are. Are Ionic Compounds Metal.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Are Ionic Compounds Metal See the study guide on the. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Covalent bonds are highly stable bonds. Are Ionic Compounds Metal.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps Are Ionic Compounds Metal Covalent bonds are highly stable bonds with. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic compounds contain both cations and. Are Ionic Compounds Metal.
From en.wikipedia.org
Ionic bonding Wikipedia Are Ionic Compounds Metal Binary ionic compounds are composed of just two elements: Covalent bonds are highly stable bonds with. See the study guide on the. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic bond, type of linkage. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Ions and Ionic Compounds PowerPoint Presentation, free download Are Ionic Compounds Metal A metal (which forms the cations) and a nonmetal. The formation of ionic compounds. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Covalent bonds are highly stable bonds with. Ionic compounds contain both cations and anions. Are Ionic Compounds Metal.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Are Ionic Compounds Metal Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Binary ionic compounds are composed of just two elements: See the study guide on the. Such a bond forms when the valence. Ionic compounds tend to be. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Binary Ionic Compounds PowerPoint Presentation, free download Are Ionic Compounds Metal A metal (which forms the cations) and a nonmetal. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. See. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download Are Ionic Compounds Metal Binary ionic compounds are composed of just two elements: In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Such a bond forms when the valence. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and. Are Ionic Compounds Metal.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Are Ionic Compounds Metal A metal (which forms the cations) and a nonmetal. Covalent bonds are highly stable bonds with. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Such a bond forms when the valence. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. Binary ionic compounds are composed. Are Ionic Compounds Metal.
From www.breakingatom.com
Ionic Properties Are Ionic Compounds Metal The formation of ionic compounds. See the study guide on the. A metal (which forms the cations) and a nonmetal. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Such a bond forms when the valence. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. In covalent compounds,. Are Ionic Compounds Metal.
From sciencenotes.org
Ionic Compound Properties Are Ionic Compounds Metal See the study guide on the. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Such a bond forms when the valence. Binary ionic compounds are composed of just two elements: Ionic compounds contain both cations and anions in. Are Ionic Compounds Metal.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Are Ionic Compounds Metal See the study guide on the. A metal (which forms the cations) and a nonmetal. Such a bond forms when the valence. Binary ionic compounds are composed of just two elements: Covalent bonds are highly stable bonds with. The formation of ionic compounds. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Ionic compounds have high melting. Are Ionic Compounds Metal.
From www.youtube.com
PROPERTIES OF IONIC COMPOUNDS AND OCCURRENCE OF METALS YouTube Are Ionic Compounds Metal Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. The formation of ionic compounds. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Covalent bonds are highly stable bonds with. Such a bond forms when the valence. Ionic compounds have high melting and boiling points, so they are. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Names and Formulas Ionic Compounds Binary Main Group M & NM Using Are Ionic Compounds Metal See the study guide on the. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. The formation of ionic compounds. Covalent bonds are highly stable bonds with. Ionic compound, any of a large group of chemical compounds consisting of. Are Ionic Compounds Metal.
From www.slideserve.com
PPT What information do the name and formula of an ionic compound Are Ionic Compounds Metal Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. The formation of ionic compounds. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. See the study guide on the. Binary ionic compounds are composed of just two elements: Ionic compounds tend to be crystalline structures. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Ion Charge and the Formulas of Ionic Compounds PowerPoint Are Ionic Compounds Metal In covalent compounds, electrons are shared between bonded atoms and are simultaneously. A metal (which forms the cations) and a nonmetal. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. Ionic compound, any of. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download Are Ionic Compounds Metal See the study guide on the. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Such a bond forms when the valence. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Binary ionic compounds are composed of just two elements: Molecular compounds form between nonmetals and nonmetals, while. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download Are Ionic Compounds Metal Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compounds contain both cations and anions in a ratio that results in no net electrical charge. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. A metal (which forms the cations) and a nonmetal. The. Are Ionic Compounds Metal.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Are Ionic Compounds Metal Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. In covalent compounds, electrons are shared between bonded atoms and are simultaneously. The formation of ionic compounds. Binary ionic compounds are composed of just two elements: Covalent bonds are highly stable bonds with. Molecular compounds form between nonmetals and nonmetals, while ionic compounds. Are Ionic Compounds Metal.
From www.youtube.com
Ionic Compounds and multivalent metals YouTube Are Ionic Compounds Metal A metal (which forms the cations) and a nonmetal. Covalent bonds are highly stable bonds with. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. See the study guide on the. Ionic bond, type of linkage. Are Ionic Compounds Metal.
From www.youtube.com
How To Name Ionic Compounds With Transition Metals YouTube Are Ionic Compounds Metal Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. The formation of ionic compounds. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. See the study guide on the. A metal (which forms the cations) and a nonmetal. Ionic compounds contain both cations and anions in. Are Ionic Compounds Metal.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download Are Ionic Compounds Metal In covalent compounds, electrons are shared between bonded atoms and are simultaneously. Ionic compounds tend to be crystalline structures with high melting points that are water soluble. Ionic compound, any of a large group of chemical compounds consisting of oppositely charged ions, wherein electron. See the study guide on the. A metal (which forms the cations) and a nonmetal. Molecular. Are Ionic Compounds Metal.