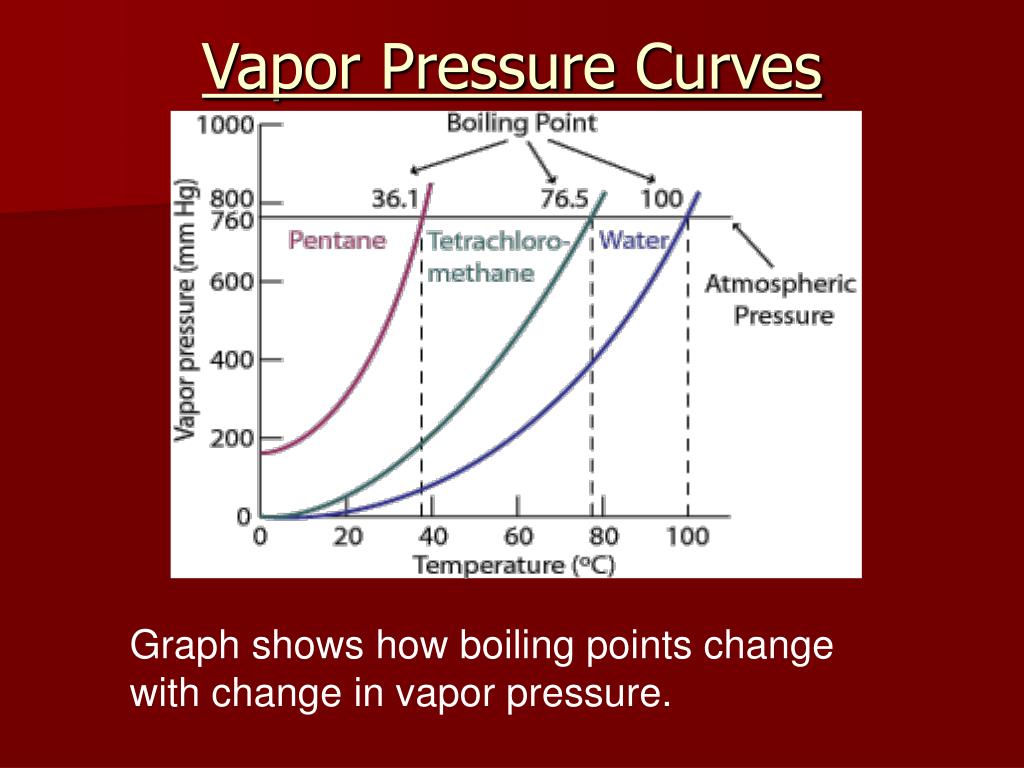
Boiling Point Definition Vapour Pressure . Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to. Therefore, the boiling point of a liquid depends on. At a pressure greater than 1 atm, water boils at a temperature. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid.
from www.slideserve.com
At a pressure greater than 1 atm, water boils at a temperature. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The change from a liquid phase to. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Therefore, the boiling point of a liquid depends on. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point.
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download
Boiling Point Definition Vapour Pressure Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Therefore, the boiling point of a liquid depends on. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. At a pressure greater than 1 atm, water boils at a temperature. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The change from a liquid phase to. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid.
From www.youtube.com
Vapour pressure boiling point composition diagrams for nonideal Boiling Point Definition Vapour Pressure A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Therefore,. Boiling Point Definition Vapour Pressure.
From www.youtube.com
Vapor Pressure & Boiling Point YouTube Boiling Point Definition Vapour Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Therefore, the boiling point of a liquid depends on. The boiling point is the temperature at which the vapor pressure of. Boiling Point Definition Vapour Pressure.
From www.youtube.com
physical sciences grade 12 vapour pressure, boiling point and melting Boiling Point Definition Vapour Pressure The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. At a pressure greater. Boiling Point Definition Vapour Pressure.
From slideplayer.com
A. Phase Changes Sublimation solid gas. Vaporization liquid gas Boiling Point Definition Vapour Pressure A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The change from a liquid phase to. Although we usually cite the normal boiling point of a liquid, the actual. Boiling Point Definition Vapour Pressure.
From apollo.nvu.vsc.edu
Saturation Vapor Pressure and the Boiling Point Boiling Point Definition Vapour Pressure A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Therefore, the boiling point of a liquid depends on. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Although we usually cite the normal boiling point of a liquid,. Boiling Point Definition Vapour Pressure.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Boiling Point Definition Vapour Pressure The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. At. Boiling Point Definition Vapour Pressure.
From www.youtube.com
Vapour Pressure and Boiling Point Explanation and notes ( LIQUID STATE Boiling Point Definition Vapour Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. At a pressure greater than 1 atm, water boils at a temperature. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Therefore, the boiling point of a liquid depends on.. Boiling Point Definition Vapour Pressure.
From www.worksheetsplanet.com
What is Boiling Point Boiling Point Definition Vapour Pressure Therefore, the boiling point of a liquid depends on. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. At a pressure greater than 1 atm, water boils at a temperature. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure.. Boiling Point Definition Vapour Pressure.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co Boiling Point Definition Vapour Pressure At a pressure greater than 1 atm, water boils at a temperature. Therefore, the boiling point of a liquid depends on. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it.. Boiling Point Definition Vapour Pressure.
From mavink.com
Water Boiling Point Pressure Chart Boiling Point Definition Vapour Pressure The change from a liquid phase to. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling is the process by which a liquid turns into a vapor when. Boiling Point Definition Vapour Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Boiling Point Definition Vapour Pressure Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Therefore, the boiling. Boiling Point Definition Vapour Pressure.
From www.pinterest.co.uk
Vapour Pressure and Boiling point Vapor, Boiling point, Chemistry class Boiling Point Definition Vapour Pressure At a pressure greater than 1 atm, water boils at a temperature. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The change from a liquid phase to. Therefore, the boiling point of a liquid depends on. Although we usually cite the normal boiling point of a liquid,. Boiling Point Definition Vapour Pressure.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Boiling Point Definition Vapour Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. At a pressure. Boiling Point Definition Vapour Pressure.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint Boiling Point Definition Vapour Pressure Therefore, the boiling point of a liquid depends on. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Although we usually cite the normal boiling point of a liquid, the. Boiling Point Definition Vapour Pressure.
From rumble.com
Vapour pressure, boiling point, properties of liquids Chemistry Boiling Point Definition Vapour Pressure The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Therefore, the boiling point of a liquid depends on. Boiling point, temperature at which the pressure exerted by the. Boiling Point Definition Vapour Pressure.
From www.youtube.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation Boiling Point Definition Vapour Pressure Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Therefore, the boiling point of a liquid depends on. The boiling point is the temperature at which the vapor pressure of. Boiling Point Definition Vapour Pressure.
From byjus.com
Describe the concept of Relative lowering of vapour pressure and Boiling Point Definition Vapour Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. At a pressure greater than 1 atm, water boils at a temperature. Therefore, the boiling point of a liquid depends on.. Boiling Point Definition Vapour Pressure.
From brainly.in
Draw vapour pressure composition diagram and corresponding boiling Boiling Point Definition Vapour Pressure At a pressure greater than 1 atm, water boils at a temperature. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to. Therefore, the boiling. Boiling Point Definition Vapour Pressure.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Boiling Point Definition Vapour Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding. Boiling Point Definition Vapour Pressure.
From www.youtube.com
Vapour Pressure and Boiling Point Relationship Vapour Pressure vs Boiling Point Definition Vapour Pressure The change from a liquid phase to. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. A liquid boils at a temperature at which its vapor pressure is equal. Boiling Point Definition Vapour Pressure.
From slideplayer.com
A. Phase Changes Sublimation solid gas. Vaporization liquid gas Boiling Point Definition Vapour Pressure Therefore, the boiling point of a liquid depends on. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. A liquid boils at a temperature at which its vapor pressure is. Boiling Point Definition Vapour Pressure.
From mungfali.com
Vapor Pressure And Boiling Point Relationship Boiling Point Definition Vapour Pressure The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. Therefore, the boiling point of a liquid depends on. Boiling is the process by which a liquid turns into a. Boiling Point Definition Vapour Pressure.
From www.youtube.com
5.2 Liquid (Vapour Pressure, Boiling Point & Normal Boiling Point Boiling Point Definition Vapour Pressure The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point.. Boiling Point Definition Vapour Pressure.
From socratic.org
What is the relation between critical temperature and boiling point or Boiling Point Definition Vapour Pressure The change from a liquid phase to. Therefore, the boiling point of a liquid depends on. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Boiling is the process by. Boiling Point Definition Vapour Pressure.
From matmake.com
Boiling Point Definition, Measurements, and Applications Boiling Point Definition Vapour Pressure Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Therefore, the boiling point of a liquid depends on. At a pressure greater than 1 atm, water boils at a temperature. The change from a liquid phase to. The boiling point is the temperature at which the vapor pressure of a. Boiling Point Definition Vapour Pressure.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube Boiling Point Definition Vapour Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. The boiling point. Boiling Point Definition Vapour Pressure.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube Boiling Point Definition Vapour Pressure Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. At a pressure greater than 1 atm, water boils at a temperature. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. The change from a liquid phase to. Boiling is the. Boiling Point Definition Vapour Pressure.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free Boiling Point Definition Vapour Pressure Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The change from a liquid phase to. Boiling point, temperature at which the pressure exerted by the surroundings upon a. Boiling Point Definition Vapour Pressure.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download Boiling Point Definition Vapour Pressure At a pressure greater than 1 atm, water boils at a temperature. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The change from a liquid phase. Boiling Point Definition Vapour Pressure.
From worksheetdbwounds.z13.web.core.windows.net
Vapor Pressure And Boiling Worksheet Boiling Point Definition Vapour Pressure At a pressure greater than 1 atm, water boils at a temperature. Therefore, the boiling point of a liquid depends on. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. The boiling. Boiling Point Definition Vapour Pressure.
From www.youtube.com
Which Compound Has a Higher Vapor Pressure? Intermolecular Force Boiling Point Definition Vapour Pressure Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. At a pressure greater than 1 atm, water boils at a temperature. The boiling point is the temperature at which the. Boiling Point Definition Vapour Pressure.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Boiling Point Definition Vapour Pressure The change from a liquid phase to. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. At a pressure greater than 1 atm, water boils at a temperature.. Boiling Point Definition Vapour Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Boiling Point Definition Vapour Pressure Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the. Boiling Point Definition Vapour Pressure.
From www.slideserve.com
PPT Chemistry 30S PowerPoint Presentation, free download ID2024732 Boiling Point Definition Vapour Pressure The change from a liquid phase to. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature at which the vapor pressure of a liquid equals. Boiling Point Definition Vapour Pressure.
From www.youtube.com
Vapour Pressure Boiling Point of LiquidsChemicalMahi YouTube Boiling Point Definition Vapour Pressure At a pressure greater than 1 atm, water boils at a temperature. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The lower the pressure of a. Boiling Point Definition Vapour Pressure.