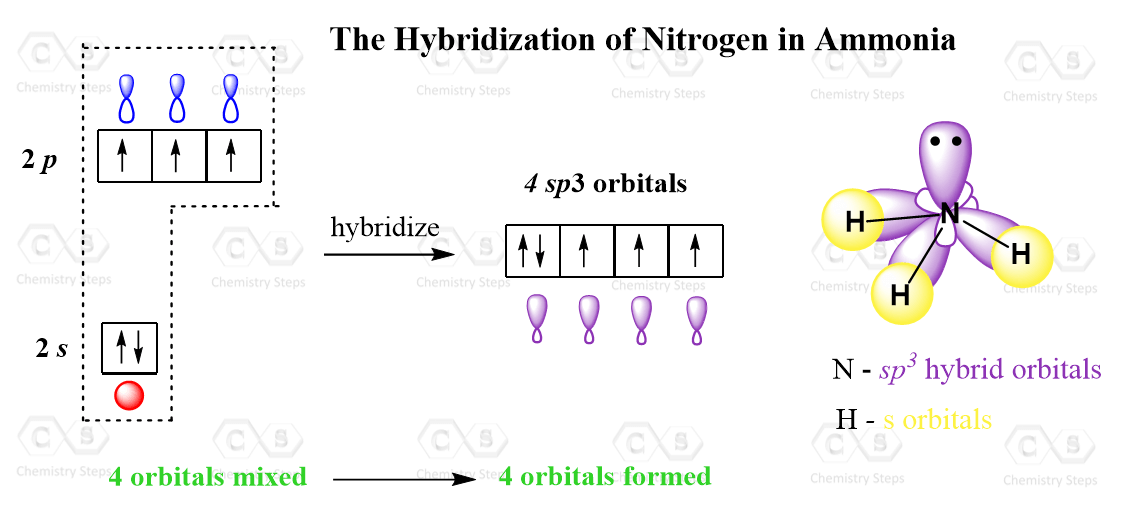
Hybridization Bonding . To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. In particular, the concept of. Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule. As you know, every atom has electron orbitals surrounding it. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the.
from general.chemistrysteps.com
Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule. To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. In particular, the concept of. As you know, every atom has electron orbitals surrounding it. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1.
Hybridization of Atomic Orbitals Chemistry Steps
Hybridization Bonding Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. As you know, every atom has electron orbitals surrounding it. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. In particular, the concept of. The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule.
From www.masterorganicchemistry.com
What Are Hybrid Orbitals? Master Organic Chemistry Hybridization Bonding Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. In particular, the concept of. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid. Hybridization Bonding.
From www.youtube.com
Sigma and Pi Bonding in Ethene (C2H4) (Hybridization) YouTube Hybridization Bonding As you know, every atom has electron orbitals surrounding it. In particular, the concept of. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be. Hybridization Bonding.
From avantecnica.qualitypoolsboulder.com
Hybridization Definition, Types, Rules, Examples Hybridization Bonding Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. The localized valence bond theory uses a process called hybridization, in which. Hybridization Bonding.
From chemistrytalk.org
Hybridization and Hybrid Orbitals ChemTalk Hybridization Bonding The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. As you know, every atom has electron orbitals surrounding it. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. Hybridization was. Hybridization Bonding.
From kanayatichemistry.blogspot.com
Hybridization K Chemistry Hybridization Bonding Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. As you know, every atom has electron orbitals surrounding it. To explain the bonding of. Hybridization Bonding.
From chemistryskills.com
Orbital Hybridization Chemistry Skills Hybridization Bonding As you know, every atom has electron orbitals surrounding it. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. In particular, the concept of. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid. Hybridization Bonding.
From www.youtube.com
CHEMISTRY 101 Valence Bond Theory hybridization in larger molecules Hybridization Bonding Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. As you know, every atom has electron orbitals surrounding it. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95%. Hybridization Bonding.
From www.youtube.com
Hybrid Orbitals explained Valence Bond Theory Orbital Hybridization Hybridization Bonding Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the. Hybridization Bonding.
From chemwiki.ucdavis.edu
10.3 Hybridization of Atomic Orbitals Chemwiki Hybridization Bonding Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. As you know, every atom has electron orbitals surrounding it. The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. To explain the bonding of carbon and other atoms that cannot fit into. Hybridization Bonding.
From collegedunia.com
Hybridization sp, sp2, sp3 & sp3d Atomic Orbitals, Properties & Examples Hybridization Bonding In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. In particular, the concept of. Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that. Hybridization Bonding.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Hybridization Bonding Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. In particular, the concept of. The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. As you know, every atom. Hybridization Bonding.
From www.youtube.com
Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp Sp2 Sp3 YouTube Hybridization Bonding In particular, the concept of. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. In chemistry, hybridisation (or hybridization) is the concept of mixing. Hybridization Bonding.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Hybridization Bonding The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Valence bond theory and hybridization can be used to explain. Hybridization Bonding.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Hybridization Bonding As you know, every atom has electron orbitals surrounding it. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. In particular, the concept of. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. Valence bond theory and hybridization can be used to. Hybridization Bonding.
From madoverchemistry.wordpress.com
62.CHEMICAL BONDING (9) Covalent Bonding(8) sp3 Hybridization Hybridization Bonding The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. As you know, every atom has electron orbitals surrounding it. In particular, the concept of. Here’s a shortcut for how to. Hybridization Bonding.
From www.slideserve.com
PPT Ch. 6—Chemical Bonding PowerPoint Presentation, free download Hybridization Bonding The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. Simply put, hybridization is the. Hybridization Bonding.
From courses.lumenlearning.com
5.5 Hybrid Atomic Orbitals General College Chemistry I Hybridization Bonding Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. In particular, the concept of. Simply put, hybridization is the way that distinct. Hybridization Bonding.
From chem.libretexts.org
Chapter 6.2 Hybrid Orbitals Chemistry LibreTexts Hybridization Bonding To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. Valence bond theory and hybridization can be used. Hybridization Bonding.
From chem.libretexts.org
10.7 Valence Bond Theory Hybridization of Atomic Orbitals Chemistry Hybridization Bonding Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. Valence bond theory and hybridization can be used to explain and/or predict the geometry of. Hybridization Bonding.
From byjus.com
Hybridization of Carbon Molecular Geometry and Bond Angles Hybridization Bonding In particular, the concept of. Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule. As you know, every atom has electron orbitals surrounding it. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases. Hybridization Bonding.
From www.brewerscience.com
Hybrid Bonding Basics What is Hybrid Bonding? Brewer Science Hybridization Bonding The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization was introduced to explain molecular structure when the valence. Hybridization Bonding.
From www.pinterest.com.mx
Orbital Hybridization "Cheat Sheet"? + Example Chemistry lessons Hybridization Bonding In particular, the concept of. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. As you know, every atom has electron orbitals surrounding it. Here’s a shortcut for how to determine the hybridization. Hybridization Bonding.
From pages.swcp.com
Parsing spdf Orbital Hybridization and Simple Bonding Hybridization Bonding Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. Valence bond theory and hybridization can be used to explain and/or predict the. Hybridization Bonding.
From www.slideserve.com
PPT Orbital Hybridization PowerPoint Presentation, free download ID Hybridization Bonding In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. In particular, the concept of. As you know, every atom has electron orbitals surrounding it. Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than. Hybridization Bonding.
From www.slideserve.com
PPT Valence Bond & hybridization PowerPoint Presentation, free Hybridization Bonding Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. To explain the bonding of carbon and other atoms that cannot. Hybridization Bonding.
From chem.libretexts.org
2.2 Valence Bond Theory Chemistry LibreTexts Hybridization Bonding Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. Valence bond theory and hybridization can be used to explain and/or predict the. Hybridization Bonding.
From socratic.org
Orbital Hybridization "Cheat Sheet"? + Example Hybridization Bonding Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a new theory called orbital hybridization will be introduced as a supplement to the valence bond theory. Simply put, hybridization is the way that distinct. Hybridization Bonding.
From www.tes.com
Hybridization and Bonding, Sigma Bonds, Pi Bonds, Grade 12 Chemistry Hybridization Bonding In particular, the concept of. Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for. Hybridization Bonding.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry Hybridization Bonding In particular, the concept of. The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. As you know, every atom has electron orbitals surrounding it. Here’s a shortcut for how to. Hybridization Bonding.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry Hybridization Bonding The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. In particular, the concept of. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Simply put, hybridization is the. Hybridization Bonding.
From www.slideserve.com
PPT Chemical Bonding and Nomenclature PowerPoint Presentation, free Hybridization Bonding The localized valence bond theory uses a process called hybridization, in which atomic orbitals that are similar in energy but. Valence bond theory and hybridization can be used to explain and/or predict the geometry of any atom in a molecule. In particular, the concept of. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly. Hybridization Bonding.
From www.geeksforgeeks.org
Hybridization Definition, Types, Rules, Examples Hybridization Bonding In particular, the concept of. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. In chemistry, hybridisation (or hybridization) is the concept. Hybridization Bonding.
From classnotes.org.in
Hybridisation Chemical Bonding and Molecular Structure, Chemistry Hybridization Bonding In particular, the concept of. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. In chemistry, hybridisation (or hybridization) is the concept. Hybridization Bonding.
From www.youtube.com
Chemistry 11Chapter 4 Chemical Bonding &Molecular Structure Hybridization Bonding Simply put, hybridization is the way that distinct atomic orbitals combine together to form identical hybrid orbitals which can participate in bonding much more favorably than unhybridized ones. Here’s a shortcut for how to determine the hybridization of an atom in a molecule that will work in at least 95% of the cases you see in org 1. To explain. Hybridization Bonding.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps Hybridization Bonding In particular, the concept of. Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the. To explain the bonding of carbon and other atoms that cannot fit into the simple valence bond theory, a. Hybridization Bonding.