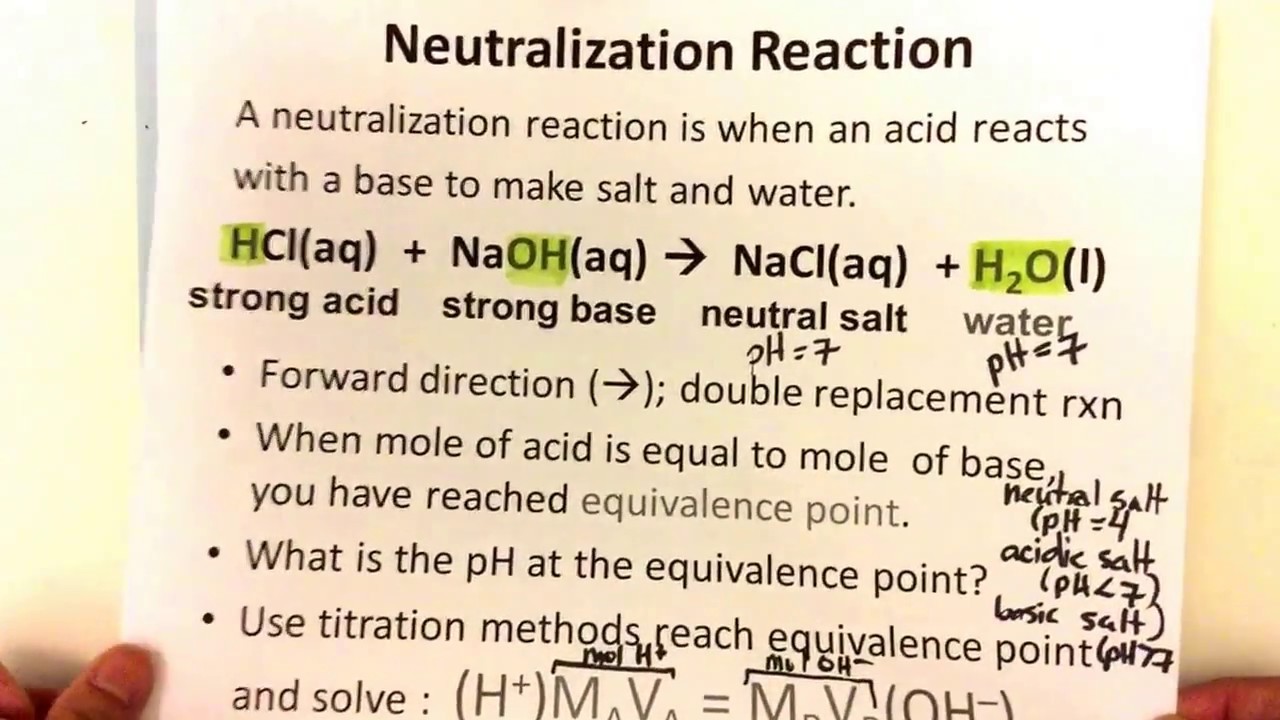
Titration Vs Neutralization . It is based on the neutralization reaction , where an. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. One of the most common and widely used ways to complete a neutralization. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known.
from www.youtube.com
In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. It is based on the neutralization reaction , where an. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. One of the most common and widely used ways to complete a neutralization.
Neutralization, Titration, and Equivalence Point YouTube
Titration Vs Neutralization One of the most common and widely used ways to complete a neutralization. One of the most common and widely used ways to complete a neutralization. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. It is based on the neutralization reaction , where an. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known.
From www.scribd.com
WEEK 9 Principles of Neutralization Titrations PDF Titration Vs Neutralization In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base. Titration Vs Neutralization.
From www.youtube.com
Acids and Bases, Titrations, and Neutralization Reactions YouTube Titration Vs Neutralization It is based on the neutralization reaction , where an. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. One of the most common and widely used ways to. Titration Vs Neutralization.
From vdocuments.mx
Acidbase titrations OH. Titration introduction Purpose Titration Vs Neutralization It is based on the neutralization reaction , where an. One of the most common and widely used ways to complete a neutralization. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with. Titration Vs Neutralization.
From www.scribd.com
Principles of Neutralization Titration Acid Titration Titration Vs Neutralization When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. It is based on the neutralization reaction , where an. One of the most common and widely used. Titration Vs Neutralization.
From www.scribd.com
3_Neutralization Titration(2) PDF Titration Vs Neutralization When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. One of the most common and widely used ways to complete a neutralization. In chemistry, neutralization or neutralisation. Titration Vs Neutralization.
From www.studypool.com
SOLUTION Analytical chemistry notes on 5 principles of neutralization Titration Vs Neutralization It is based on the neutralization reaction , where an. One of the most common and widely used ways to complete a neutralization. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it. Titration Vs Neutralization.
From www.tes.com
Acid Base Titrations, Neutralization, Equivalence Point Grade 11 Titration Vs Neutralization In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. One of the most common and widely used ways to complete a neutralization. It is based on the neutralization reaction , where an. In summary, titration is a technique used to determine the concentration of a substance. Titration Vs Neutralization.
From thenoveldifference.com
Titration and Neutralization 6 Fancy Difference Titration Vs Neutralization In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. It is based on the neutralization reaction , where an. One of. Titration Vs Neutralization.
From cesdjxor.blob.core.windows.net
Titration Neutralization Equation at Staci Arroyo blog Titration Vs Neutralization One of the most common and widely used ways to complete a neutralization. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. It is based on the neutralization reaction , where an. In summary, titration is a technique used to determine the concentration of a substance. Titration Vs Neutralization.
From www.youtube.com
11 02 Neutralization and Titration YouTube Titration Vs Neutralization When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. One of the most common and widely used ways to complete a neutralization. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. In chemistry, neutralization or neutralisation. Titration Vs Neutralization.
From cesdjxor.blob.core.windows.net
Titration Neutralization Equation at Staci Arroyo blog Titration Vs Neutralization In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. It is based on the neutralization reaction , where an. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. When a. Titration Vs Neutralization.
From www.youtube.com
Chemistry 30 Acid Base Neutralization and Titration YouTube Titration Vs Neutralization When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. One of the most common and widely used ways to complete a neutralization. It is based on the neutralization reaction , where an. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with. Titration Vs Neutralization.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Vs Neutralization It is based on the neutralization reaction , where an. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. One of the most common and widely used ways to complete a neutralization. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction. Titration Vs Neutralization.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download Titration Vs Neutralization In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. One of the most common and widely used ways to complete a neutralization. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In chemistry, neutralization or neutralisation. Titration Vs Neutralization.
From www.youtube.com
212 Exp 5 Neutralization Titration In Aqueous Medium YouTube Titration Vs Neutralization It is based on the neutralization reaction , where an. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the. Titration Vs Neutralization.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download Titration Vs Neutralization It is based on the neutralization reaction , where an. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. When a. Titration Vs Neutralization.
From slideplayer.com
Titrations!. ppt download Titration Vs Neutralization In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. It is based on the neutralization reaction , where an. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. One of the most common and widely used. Titration Vs Neutralization.
From www.youtube.com
Neutralization Reactions and Titrations YouTube Titration Vs Neutralization It is based on the neutralization reaction , where an. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. One of the most common and widely used ways to. Titration Vs Neutralization.
From exyyylzwb.blob.core.windows.net
Titration In Pharmaceutical Industry at Jamel Edwards blog Titration Vs Neutralization In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. One of the most common and widely used ways to complete a neutralization. It is based on the neutralization reaction , where an. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction. Titration Vs Neutralization.
From www.scribd.com
Principle of Neutralization Titrations PDF Acid Ph Titration Vs Neutralization In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. It is based on the neutralization reaction , where an. One of the most common and widely used. Titration Vs Neutralization.
From present5.com
Titration and AcidBase Neutralization LEARNING OBJECTIVES 11.2.2.7 Titration Vs Neutralization One of the most common and widely used ways to complete a neutralization. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. It is based on the neutralization reaction , where an. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with. Titration Vs Neutralization.
From www.youtube.com
Types of Neutralization Titrations YouTube Titration Vs Neutralization In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. When a strong base neutralizes a weak acid, the resulting solution's ph. Titration Vs Neutralization.
From www.slideserve.com
PPT Chapter 14 Principles of Neutralization Titrations PowerPoint Titration Vs Neutralization In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. One of the most common and widely used ways to complete a neutralization. It is based on the neutralization reaction , where an. When a strong base neutralizes a weak acid, the resulting solution's ph. Titration Vs Neutralization.
From ceaynemy.blob.core.windows.net
Titration Neutralization Test at Mark Rice blog Titration Vs Neutralization When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. It is based on the neutralization reaction , where an. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. One of the most common and widely used. Titration Vs Neutralization.
From www.youtube.com
AcidBase Vid3 Neutralization & Titrations YouTube Titration Vs Neutralization When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. One of the most common and widely used ways to complete a neutralization. It is based on the. Titration Vs Neutralization.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Vs Neutralization One of the most common and widely used ways to complete a neutralization. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of. Titration Vs Neutralization.
From thecontentauthority.com
Titration vs Neutralization Meaning And Differences Titration Vs Neutralization In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. It is based on the neutralization reaction , where an. In chemistry, neutralization or neutralisation (see spelling differences). Titration Vs Neutralization.
From slideplayer.com
Titration and oxidation numbers Part V ppt download Titration Vs Neutralization When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. It is based on the neutralization reaction , where an. One of the most common and widely used. Titration Vs Neutralization.
From relationshipbetween.com
Difference Between Titration And Neutralization Relationship Between Titration Vs Neutralization In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. It is based on the neutralization reaction , where an. One of. Titration Vs Neutralization.
From www.slideserve.com
PPT Neutralization and Titration PowerPoint Presentation, free Titration Vs Neutralization In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it. Titration Vs Neutralization.
From thenoveldifference.com
Titration and Neutralization 6 Fancy Difference Titration Vs Neutralization One of the most common and widely used ways to complete a neutralization. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. It is based on the. Titration Vs Neutralization.
From www.youtube.com
Neutralization, Titration, and Equivalence Point YouTube Titration Vs Neutralization One of the most common and widely used ways to complete a neutralization. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. It is based on the neutralization reaction. Titration Vs Neutralization.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download Titration Vs Neutralization In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. One of the most common and widely used ways to complete a neutralization. It is based on the neutralization reaction. Titration Vs Neutralization.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Vs Neutralization In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. One of the most common and widely used ways to complete a neutralization. It is based on the neutralization reaction , where an. When a strong base neutralizes a weak acid, the resulting solution's ph will be. Titration Vs Neutralization.
From www.differencebetween.com
Difference Between Titration and Neutralization Compare the Titration Vs Neutralization When a strong base neutralizes a weak acid, the resulting solution's ph will be greater than 7. In summary, titration is a technique used to determine the concentration of a substance in a solution by reacting it with a solution of known. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base. Titration Vs Neutralization.