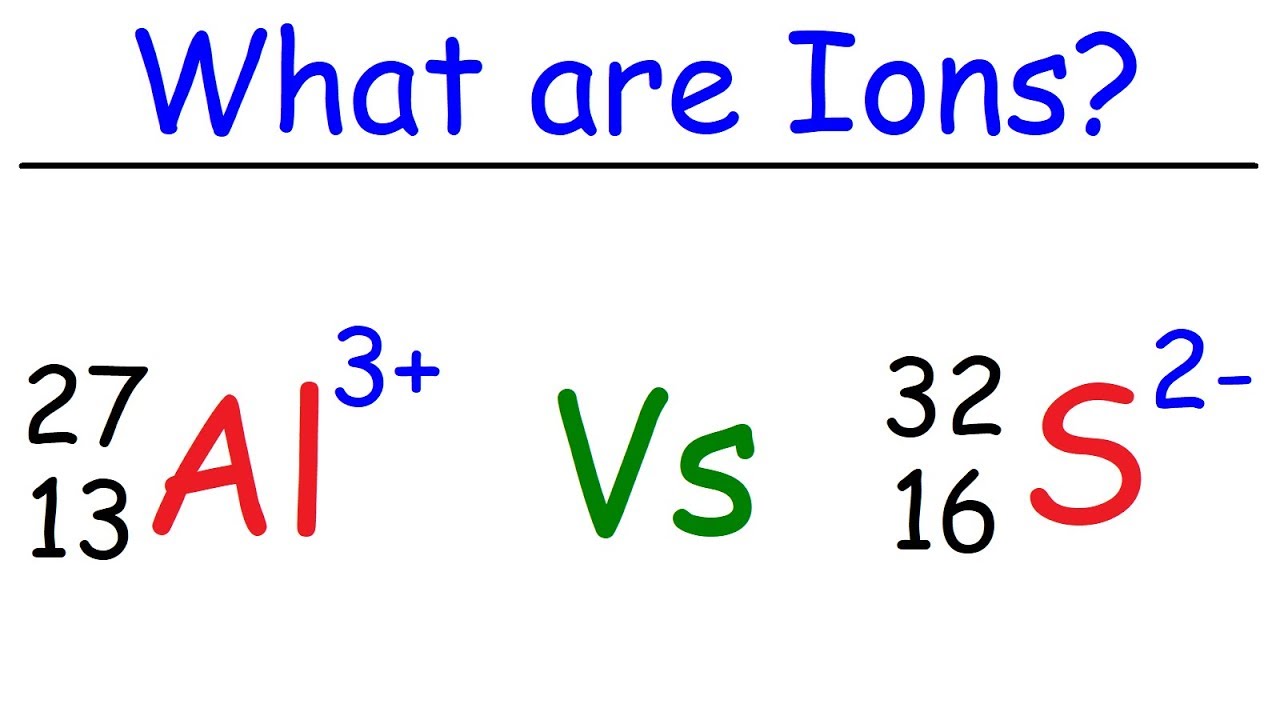What Is Ion Name . Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Thus, na + is the sodium ion, al 3 + is the. A helium atom that has lost or gained an electron is an ion. After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. Electron transfer between lithium (li) and fluorine (f). After learning a few more details about the names of individual ions, you will be a step away from. Au 3+ gold(iii) / auric: The name of a monatomic cation is simply the name of the element followed by the word ion. We begin with binary ionic compounds, which contain only two elements. Au + gold(i) / aurous: They have a giant lattice structure with strong ionic bonds. The procedure for naming such compounds is outlined in figure 2.10. Forming an ionic bond, li and f become li + and f − ions. Use the rules for naming ionic compounds. To use the rules for naming ionic compounds.
from www.youtube.com
Forming an ionic bond, li and f become li + and f − ions. A helium atom that has lost or gained an electron is an ion. Use the rules for naming ionic compounds. After learning a few more details about the names of individual ions, you will be a step away from. They have a giant lattice structure with strong ionic bonds. Au 3+ gold(iii) / auric: Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. To use the rules for naming ionic compounds. The name of a monatomic cation is simply the name of the element followed by the word ion. Thus, na + is the sodium ion, al 3 + is the.
What is an Ion? YouTube
What Is Ion Name After learning a few more details about the names of individual ions, you will be a step away from. They have a giant lattice structure with strong ionic bonds. Thus, na + is the sodium ion, al 3 + is the. The procedure for naming such compounds is outlined in figure 2.10. We begin with binary ionic compounds, which contain only two elements. A helium atom that has lost or gained an electron is an ion. Forming an ionic bond, li and f become li + and f − ions. Au + gold(i) / aurous: Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. To use the rules for naming ionic compounds. After learning a few more details about the names of individual ions, you will be a step away from. The name of a monatomic cation is simply the name of the element followed by the word ion. Use the rules for naming ionic compounds. Au 3+ gold(iii) / auric: After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. Electron transfer between lithium (li) and fluorine (f).
From saylordotorg.github.io
Naming Ionic Compounds What Is Ion Name The name of a monatomic cation is simply the name of the element followed by the word ion. To use the rules for naming ionic compounds. Au 3+ gold(iii) / auric: A helium atom that has lost or gained an electron is an ion. Au + gold(i) / aurous: The procedure for naming such compounds is outlined in figure 2.10.. What Is Ion Name.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry What Is Ion Name After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. The procedure for naming such compounds is outlined in figure 2.10. They have a giant lattice structure with strong ionic bonds. Thus, na + is the sodium ion, al 3 + is the. To use. What Is Ion Name.
From www.youtube.com
What is an Ion? YouTube What Is Ion Name A helium atom that has lost or gained an electron is an ion. They have a giant lattice structure with strong ionic bonds. After learning a few more details about the names of individual ions, you will be a step away from. Electron transfer between lithium (li) and fluorine (f). Learn about and revise the structure of atoms, atoms and. What Is Ion Name.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry What Is Ion Name Au + gold(i) / aurous: To use the rules for naming ionic compounds. Au 3+ gold(iii) / auric: Electron transfer between lithium (li) and fluorine (f). Use the rules for naming ionic compounds. Forming an ionic bond, li and f become li + and f − ions. Thus, na + is the sodium ion, al 3 + is the. After. What Is Ion Name.
From www.youtube.com
Naming Ions How to Write the Names for Ions YouTube What Is Ion Name Au + gold(i) / aurous: To use the rules for naming ionic compounds. We begin with binary ionic compounds, which contain only two elements. The name of a monatomic cation is simply the name of the element followed by the word ion. Use the rules for naming ionic compounds. Electron transfer between lithium (li) and fluorine (f). Forming an ionic. What Is Ion Name.
From www.expii.com
Ions — Definition & Overview Expii What Is Ion Name The procedure for naming such compounds is outlined in figure 2.10. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. After learning a few more details about the names of individual ions, you will be a step away from. They have a giant lattice structure with strong ionic bonds. The name. What Is Ion Name.
From engineeringoperations.blogspot.com
Monoatomic and polyatomic ion names and formulae BASIC CHEMICAL What Is Ion Name Electron transfer between lithium (li) and fluorine (f). We begin with binary ionic compounds, which contain only two elements. Au 3+ gold(iii) / auric: After learning a few more details about the names of individual ions, you will be a step away from. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined. What Is Ion Name.
From webmis.highland.cc.il.us
Naming Ionic Compounds What Is Ion Name A helium atom that has lost or gained an electron is an ion. The procedure for naming such compounds is outlined in figure 2.10. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Use the rules for naming ionic compounds. Au + gold(i) / aurous: They have a giant lattice structure. What Is Ion Name.
From www.slideserve.com
PPT Chemical Names of Ionic Compounds PowerPoint Presentation, free What Is Ion Name After learning a few more details about the names of individual ions, you will be a step away from. Forming an ionic bond, li and f become li + and f − ions. A helium atom that has lost or gained an electron is an ion. The name of a monatomic cation is simply the name of the element followed. What Is Ion Name.
From inspiritvr.com
Polyatomic Ions Naming and Formulas Study Guide Inspirit What Is Ion Name After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. Use the rules for naming ionic compounds. A helium atom that has lost or gained an electron is an ion. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse. What Is Ion Name.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 What Is Ion Name The procedure for naming such compounds is outlined in figure 2.10. Forming an ionic bond, li and f become li + and f − ions. The name of a monatomic cation is simply the name of the element followed by the word ion. After learning a few more details about the names of individual ions, you will be one step. What Is Ion Name.
From www.youtube.com
Naming Simple Ionic Compounds YouTube What Is Ion Name Forming an ionic bond, li and f become li + and f − ions. Thus, na + is the sodium ion, al 3 + is the. Electron transfer between lithium (li) and fluorine (f). The name of a monatomic cation is simply the name of the element followed by the word ion. Au + gold(i) / aurous: The procedure for. What Is Ion Name.
From www.pinterest.at
Common Polyatomic ions Teaching chemistry, Chemistry lessons What Is Ion Name We begin with binary ionic compounds, which contain only two elements. Forming an ionic bond, li and f become li + and f − ions. Thus, na + is the sodium ion, al 3 + is the. Au + gold(i) / aurous: Au 3+ gold(iii) / auric: Learn about and revise the structure of atoms, atoms and isotopes and ions. What Is Ion Name.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry What Is Ion Name To use the rules for naming ionic compounds. Forming an ionic bond, li and f become li + and f − ions. After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. Thus, na + is the sodium ion, al 3 + is the. The. What Is Ion Name.
From inorganicnomenclaturerice.weebly.com
Naming Ionic Compounds What Is Ion Name Use the rules for naming ionic compounds. Au 3+ gold(iii) / auric: To use the rules for naming ionic compounds. The name of a monatomic cation is simply the name of the element followed by the word ion. Forming an ionic bond, li and f become li + and f − ions. Electron transfer between lithium (li) and fluorine (f).. What Is Ion Name.
From scientifictutor.org
Chem Ions Scientific Tutor What Is Ion Name Use the rules for naming ionic compounds. Au 3+ gold(iii) / auric: Au + gold(i) / aurous: After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. A helium atom that has lost or gained an electron is an ion. Learn about and revise the. What Is Ion Name.
From www.pathwaystochemistry.com
Naming Simple Ionic Compounds Pathways to Chemistry What Is Ion Name Forming an ionic bond, li and f become li + and f − ions. Au + gold(i) / aurous: Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. We begin with binary ionic compounds, which contain only two elements. The procedure for naming such compounds is outlined in figure 2.10. Au. What Is Ion Name.
From printablegnusobimab1.z22.web.core.windows.net
Names And Formulas Of Polyatomic Ions What Is Ion Name Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Use the rules for naming ionic compounds. To use the rules for naming ionic compounds. Electron transfer between lithium (li) and fluorine (f). After learning a few more details about the names of individual ions, you will be a step away from.. What Is Ion Name.
From www.templateroller.com
Common Ions Chart Download Printable PDF Templateroller What Is Ion Name Au + gold(i) / aurous: After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. Forming an ionic bond, li and f become li + and f − ions. They have a giant lattice structure with strong ionic bonds. The procedure for naming such compounds. What Is Ion Name.
From superhumanacademy.com
How to Memorize Polyatomic Ions & Chemical Formulas SuperHuman Academy What Is Ion Name After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. The name of a monatomic cation is simply the name of the element followed by the word ion. Forming an ionic bond, li and f become li + and f − ions. We begin with. What Is Ion Name.
From www.tes.com
Polyatomic Ions Lessons TES What Is Ion Name A helium atom that has lost or gained an electron is an ion. After learning a few more details about the names of individual ions, you will be a step away from. After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. Learn about and. What Is Ion Name.
From www.pinterest.com.au
polyatomicions A list of the names and formulas of some common What Is Ion Name Thus, na + is the sodium ion, al 3 + is the. The name of a monatomic cation is simply the name of the element followed by the word ion. Au 3+ gold(iii) / auric: They have a giant lattice structure with strong ionic bonds. Use the rules for naming ionic compounds. We begin with binary ionic compounds, which contain. What Is Ion Name.
From www.eonslearning.org
Common Ion Sheet EONS LEARNING What Is Ion Name The name of a monatomic cation is simply the name of the element followed by the word ion. They have a giant lattice structure with strong ionic bonds. The procedure for naming such compounds is outlined in figure 2.10. Au 3+ gold(iii) / auric: Forming an ionic bond, li and f become li + and f − ions. Au +. What Is Ion Name.
From www.slideserve.com
PPT 1 Name the ions formed by these elements and classify them as What Is Ion Name They have a giant lattice structure with strong ionic bonds. Electron transfer between lithium (li) and fluorine (f). The name of a monatomic cation is simply the name of the element followed by the word ion. After learning a few more details about the names of individual ions, you will be a step away from. Forming an ionic bond, li. What Is Ion Name.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps What Is Ion Name After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. We begin with binary ionic compounds, which contain only two elements. Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. They have a giant lattice structure. What Is Ion Name.
From www.wikihow.com
3 Ways to Name Ions wikiHow What Is Ion Name The name of a monatomic cation is simply the name of the element followed by the word ion. They have a giant lattice structure with strong ionic bonds. The procedure for naming such compounds is outlined in figure 2.10. After learning a few more details about the names of individual ions, you will be one step away from knowing how. What Is Ion Name.
From www.slideserve.com
PPT Common Polyatomic Ions PowerPoint Presentation, free download What Is Ion Name The name of a monatomic cation is simply the name of the element followed by the word ion. Thus, na + is the sodium ion, al 3 + is the. Forming an ionic bond, li and f become li + and f − ions. Use the rules for naming ionic compounds. After learning a few more details about the names. What Is Ion Name.
From www.compoundchem.com
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest What Is Ion Name They have a giant lattice structure with strong ionic bonds. After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. Forming an ionic bond, li and f become li + and f − ions. Au + gold(i) / aurous: We begin with binary ionic compounds,. What Is Ion Name.
From www.youtube.com
Naming Ionic Compounds, a tutorial Crash Chemistry Academy YouTube What Is Ion Name The procedure for naming such compounds is outlined in figure 2.10. Forming an ionic bond, li and f become li + and f − ions. After learning a few more details about the names of individual ions, you will be one step away from knowing how to name ionic compounds. To use the rules for naming ionic compounds. After learning. What Is Ion Name.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free What Is Ion Name Thus, na + is the sodium ion, al 3 + is the. We begin with binary ionic compounds, which contain only two elements. To use the rules for naming ionic compounds. The procedure for naming such compounds is outlined in figure 2.10. Electron transfer between lithium (li) and fluorine (f). Au + gold(i) / aurous: A helium atom that has. What Is Ion Name.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry What Is Ion Name The procedure for naming such compounds is outlined in figure 2.10. We begin with binary ionic compounds, which contain only two elements. Forming an ionic bond, li and f become li + and f − ions. The name of a monatomic cation is simply the name of the element followed by the word ion. Use the rules for naming ionic. What Is Ion Name.
From www.youtube.com
How to Memorize The Polyatomic Ions Formulas, Charges, Naming What Is Ion Name The procedure for naming such compounds is outlined in figure 2.10. We begin with binary ionic compounds, which contain only two elements. After learning a few more details about the names of individual ions, you will be a step away from. Use the rules for naming ionic compounds. To use the rules for naming ionic compounds. After learning a few. What Is Ion Name.
From www.sliderbase.com
Naming Ionic Compounds What Is Ion Name After learning a few more details about the names of individual ions, you will be a step away from. Au 3+ gold(iii) / auric: Use the rules for naming ionic compounds. We begin with binary ionic compounds, which contain only two elements. They have a giant lattice structure with strong ionic bonds. The procedure for naming such compounds is outlined. What Is Ion Name.
From www.compoundchem.com
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest What Is Ion Name Learn about and revise the structure of atoms, atoms and isotopes and ions with gcse bitesize combined science. Au 3+ gold(iii) / auric: The name of a monatomic cation is simply the name of the element followed by the word ion. Thus, na + is the sodium ion, al 3 + is the. Electron transfer between lithium (li) and fluorine. What Is Ion Name.
From ubicaciondepersonas.cdmx.gob.mx
Ion Names, Formulas And Charges Chart Flinn Scientific What Is Ion Name Thus, na + is the sodium ion, al 3 + is the. The procedure for naming such compounds is outlined in figure 2.10. Au 3+ gold(iii) / auric: After learning a few more details about the names of individual ions, you will be a step away from. Electron transfer between lithium (li) and fluorine (f). After learning a few more. What Is Ion Name.