Canada Ita Medical Device . Applications for medical device investigational testing authorizations applications for medical device itas : This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the.
from present5.com
Applications for medical device investigational testing authorizations applications for medical device itas : This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for.
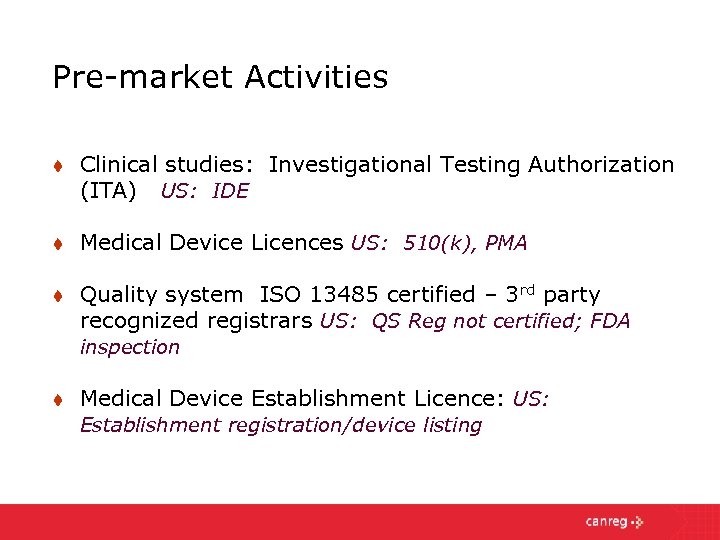
Entering the North American Market The Regulatory Landscape
Canada Ita Medical Device This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. Applications for medical device investigational testing authorizations applications for medical device itas : This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for.
From www.slideshare.net
Canada medical device approval chart EMERGO Canada Ita Medical Device Applications for medical device investigational testing authorizations applications for medical device itas : Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy. Canada Ita Medical Device.
From www.immiboards.com
Documents required for Job Verification after ITA Canada Canada Ita Medical Device This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and. Canada Ita Medical Device.
From learnwithanjali.com
From India to CanadaITA as an FSW Express Entry Learn with Anjali Canada Ita Medical Device • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. An investigational testing authorization (“ita”) is required to. Canada Ita Medical Device.
From present5.com
Entering the North American Market The Regulatory Landscape Canada Ita Medical Device This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. This guidance document is intended to assist manufacturers and importers with organizing. Canada Ita Medical Device.
From roundworldimmigration.com
Canada ITA Process [ Infographics ] Canada Visa Process Canada Ita Medical Device This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. Applications for medical device investigational testing authorizations applications for medical device itas. Canada Ita Medical Device.
From medicaldevices.freyrsolutions.com
Health Canada Medical Device Registration, Health Canada Medical Device Canada Ita Medical Device This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and. Canada Ita Medical Device.
From www.youtube.com
After receiving ITA. Post ITA process for Canada Immigration YouTube Canada Ita Medical Device This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. This page was developed to share resources related. Canada Ita Medical Device.
From www.ainit.net
ITA AINiT Consultancy Services Canada Ita Medical Device This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. Applications for medical device investigational testing authorizations applications for medical device itas : This guidance document is intended to assist manufacturers. Canada Ita Medical Device.
From www.universaladviser.com
How to get Canada PR visa? Step by step guide Canada Ita Medical Device This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. An investigational testing. Canada Ita Medical Device.
From joillrflz.blob.core.windows.net
Check Application Status Tr To Pr at Nathan Ludwig blog Canada Ita Medical Device This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. • medical device already on the market being evaluated for new intended uses, new populations, new. Canada Ita Medical Device.
From www.itamed.com
Home Health Care & Orthopedic Sports Medical Products ITAMED Canada Ita Medical Device This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. Applications for medical device investigational testing authorizations applications for medical device itas : This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. Investigational testing authorization. Canada Ita Medical Device.
From www.youtube.com
How an ITA letter for CANADA PR looks like ?? !!! YouTube Canada Ita Medical Device This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. This page was. Canada Ita Medical Device.
From www.canadafornewbies.com
How to complete your Invitation to Apply 7 steps from ITA to CoPR Canada Ita Medical Device Applications for medical device investigational testing authorizations applications for medical device itas : This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. • medical device already on the market being evaluated for new intended uses, new populations, new. Canada Ita Medical Device.
From www.canada.ca
Approved in 2020 Medical devices Canada.ca Canada Ita Medical Device Applications for medical device investigational testing authorizations applications for medical device itas : Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. This draft guidance document. Canada Ita Medical Device.
From 123bike.biz
ircc medical exam DrBeckmann Canada Ita Medical Device • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for. Investigational testing authorization (ita) issued by health canada, allows for the testing of class. Canada Ita Medical Device.
From www.settler.ca
ITA or Invitation to Apply in Canadian Immigration Canada Ita Medical Device This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. • medical device already on the market being evaluated for new. Canada Ita Medical Device.
From ocscglobal.com
Canada Invitation to Apply (ITA) for Healthcare Occupations Aanchal Canada Ita Medical Device This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. This page was developed to share resources related. Canada Ita Medical Device.
From cosmosimmigration.com
What is ITA and how to receive it through express entry? Canada Ita Medical Device This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. Applications for medical device investigational testing authorizations applications for medical device itas : This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device,. Canada Ita Medical Device.
From imdsrl.com
Nasce Italian Medical Device IMD Italian Medical Devices Canada Ita Medical Device Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. • medical device already on the market. Canada Ita Medical Device.
From www.pinterest.com
D.B.Medical Representative & Consultancy Devices and Biotechnology Canada Ita Medical Device • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv. Canada Ita Medical Device.
From www.visaplace.com
How to Get an ITA Canada Ita Medical Device • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv. Canada Ita Medical Device.
From www.slideserve.com
PPT ITA Trade Facilitation Activities and Review of Key Medical Canada Ita Medical Device This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. This draft guidance document reflects health canada’s. Canada Ita Medical Device.
From www.youtube.com
ITAMED Co Manufacturer of medical support products for a healthy Canada Ita Medical Device An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. Applications for medical device investigational testing authorizations applications for medical device itas :. Canada Ita Medical Device.
From www.walmart.com
ITAMED Breathable Elastic Rib Brace for Men RSM223, Large Canada Ita Medical Device This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita. Canada Ita Medical Device.
From www.canada.ca
Applications for Medical Device Investigational Testing Authorizations Canada Ita Medical Device Applications for medical device investigational testing authorizations applications for medical device itas : This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. • medical device already on the market being evaluated for new intended uses, new populations, new. Canada Ita Medical Device.
From www.slideserve.com
PPT ITA Trade Facilitation Activities and Review of Key Medical Canada Ita Medical Device Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. This page was developed to share resources related to the investigational testing provisions under the medical devices. Canada Ita Medical Device.
From www.canada.ca
Draft Guidance Document Applications for Medical Device Canada Ita Medical Device • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita). Canada Ita Medical Device.
From ocscglobal.com
Invitation To Apply (ITA) under Canada Express Entry Draw 219 OCSC Canada Ita Medical Device • medical device already on the market being evaluated for new intended uses, new populations, new materials or design changes •. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita). Canada Ita Medical Device.
From www.joharidigital.com
Health Canada Approval Process for Medical Devices StepbyStep Guide Canada Ita Medical Device Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that. Canada Ita Medical Device.
From omcmedical.com
Health Canada Medical Device Listing OMC Medical Canada Ita Medical Device This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for. Applications for medical device. Canada Ita Medical Device.
From distrettobiomedicale.it
ITA Canada Offerta per materiale di laboratorio Elettrofisiologia Canada Ita Medical Device Applications for medical device investigational testing authorizations applications for medical device itas : An investigational testing authorization (“ita”) is required to sell or import a class ii, iii, or iv medical device that is to be used for. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human.. Canada Ita Medical Device.
From bouncemagazine.co.uk
Canada Immigration Express Entry Take Steps towards a Bright Future Canada Ita Medical Device This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita. Canada Ita Medical Device.
From ita-medical.de
ITA medical START Canada Ita Medical Device This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. Investigational testing authorization (ita) issued by health canada, allows for the testing of class ii, iii, and iv medical devices with human. An investigational testing authorization (“ita”) is required. Canada Ita Medical Device.
From www.regdesk.co
Recent Changes to Medical Device Regulations in Canada RegDesk Canada Ita Medical Device This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. Applications for medical device investigational testing authorizations applications for medical device itas : This draft guidance document reflects health canada’s current thinking on investigational testing authorizations (ita) for medical devices and may be subject to changes as policy develops. • medical device. Canada Ita Medical Device.
From www.researchgate.net
1Medical device risk management strategy (Adapted from ISO 14971 Canada Ita Medical Device This guidance document is intended to assist manufacturers and importers with organizing and submitting an ita application to conduct investigational testing of a class ii, iii or iv device, by the. This page was developed to share resources related to the investigational testing provisions under the medical devices regulations. Applications for medical device investigational testing authorizations applications for medical device. Canada Ita Medical Device.