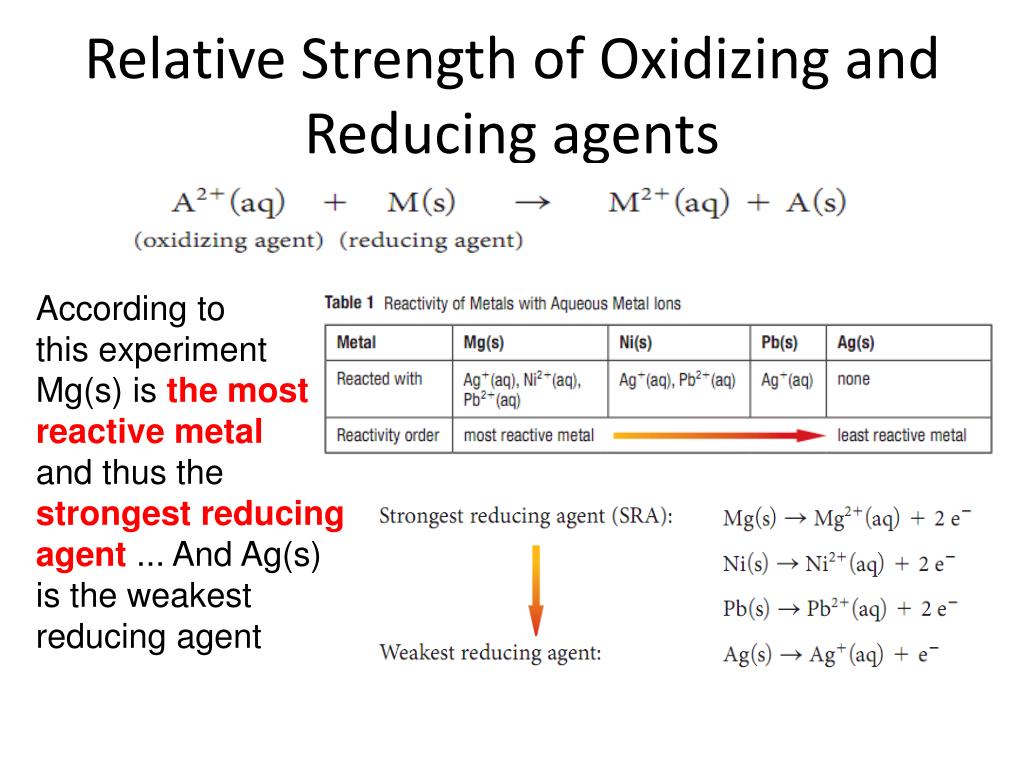
Which Of The Following Metals Is The Best Reducing Agent . A reducing agent is oxidized, because it loses electrons in the redox reaction. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. A) ni b) mg c) fe d) co 2. The standard reduction potentials can be. Which of the following metal cations is the best. A reducing agent loses electrons and is oxidized in a chemical reaction. The correct answer for reducing agent is barium (ba), as can be read from the table you provided. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. Which of the following metals is the best reducing agent? A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly easily.
from www.slideserve.com
A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. A reducing agent is oxidized, because it loses electrons in the redox reaction. The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. Which of the following metals is the best reducing agent? The standard reduction potentials can be. A) ni b) mg c) fe d) co 2. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. Which of the following metal cations is the best. A reducing agent loses electrons and is oxidized in a chemical reaction.
PPT Electrochemistry PowerPoint Presentation, free download ID3527121
Which Of The Following Metals Is The Best Reducing Agent Which of the following metal cations is the best. The correct answer for reducing agent is barium (ba), as can be read from the table you provided. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). A) ni b) mg c) fe d) co 2. The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. A reducing agent is oxidized, because it loses electrons in the redox reaction. Which of the following metal cations is the best. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. A reducing agent loses electrons and is oxidized in a chemical reaction. Which of the following metals is the best reducing agent? All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly easily. The standard reduction potentials can be. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution.
From jeanaxschneider.blogspot.com
Are Metals Reducing Agents JeanaxSchneider Which Of The Following Metals Is The Best Reducing Agent Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). The. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Which of the following is the best reducing agent? Which Of The Following Metals Is The Best Reducing Agent The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Which of the following metals is the best reducing agent? All metals have low ionization energies and are relatively electropositive,. Which Of The Following Metals Is The Best Reducing Agent.
From sciencenotes.org
Reducing Agent (Reductant) Definition and Examples Which Of The Following Metals Is The Best Reducing Agent A reducing agent is oxidized, because it loses electrons in the redox reaction. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). The strongest reducing agents are the alkali metals (group 1) as they. Which Of The Following Metals Is The Best Reducing Agent.
From www.slideserve.com
PPT Relative Strengths of Oxidizing and Reducing Agents PowerPoint Which Of The Following Metals Is The Best Reducing Agent A reducing agent is oxidized, because it loses electrons in the redox reaction. A) ni b) mg c) fe d) co 2. Which of the following metals is the best reducing agent? The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. Lithium metal is therefore the strongest reductant (most. Which Of The Following Metals Is The Best Reducing Agent.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3527121 Which Of The Following Metals Is The Best Reducing Agent All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly easily. The correct answer for reducing agent is barium (ba), as can be read from the table you provided. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). The strongest reducing. Which Of The Following Metals Is The Best Reducing Agent.
From chemistrytalk.org
Common Oxidizing Agents & Reducing Agents ChemTalk Which Of The Following Metals Is The Best Reducing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. The correct answer for reducing agent is barium (ba), as can be read from the table you provided. Which of the following metals is the best reducing agent? Barium releases a lot of energy when oxidized to bax2+ b a. Which Of The Following Metals Is The Best Reducing Agent.
From www.slideserve.com
PPT Relative Strengths of Oxidizing and Reducing Agents PowerPoint Which Of The Following Metals Is The Best Reducing Agent The standard reduction potentials can be. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). The correct answer for reducing agent is barium (ba), as can be read from the table you provided. The strongest reducing agents are the alkali metals (group 1) as they have low. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Which of the following is the best reducing agent? Which Of The Following Metals Is The Best Reducing Agent Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). A reducing agent is oxidized, because it loses electrons in the redox reaction. The standard reduction potentials can be. Which of the following metal cations is the best. Examples of reducing agents include the earth metals, formic acid,. Which Of The Following Metals Is The Best Reducing Agent.
From www.numerade.com
SOLVED The metals A, B, C, and D react in the following manners A+B Which Of The Following Metals Is The Best Reducing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. Which of the following metals is the best reducing agent? The standard reduction potentials can be. A reducing agent loses electrons and is. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Part A Which of the following metals is the best Which Of The Following Metals Is The Best Reducing Agent Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. A reducing agent loses electrons and is oxidized in a chemical reaction. The correct answer for reducing agent is barium (ba), as can be read from the table you provided. Which of the following metals is the best reducing agent? The standard reduction potentials can be. Barium. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved 1. (5 pts) Which of the following substances is the Which Of The Following Metals Is The Best Reducing Agent The standard reduction potentials can be. The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly easily. The correct answer for reducing agent is barium (ba), as can be read from the table you. Which Of The Following Metals Is The Best Reducing Agent.
From www.youtube.com
Which of the following elements acts as the best reducing agent? 10 Which Of The Following Metals Is The Best Reducing Agent A reducing agent is oxidized, because it loses electrons in the redox reaction. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. A) ni b) mg c). Which Of The Following Metals Is The Best Reducing Agent.
From www.numerade.com
The metals A, B, C, and D react in the following manners A+B^+… B+A Which Of The Following Metals Is The Best Reducing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. Which of the following metals is the best reducing agent? The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose. Which Of The Following Metals Is The Best Reducing Agent.
From chemistry.stackexchange.com
chemistry Best oxidizing and reducing agents Na, Zn^2+, Ba Which Of The Following Metals Is The Best Reducing Agent Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. A) ni b) mg c) fe d) co 2. The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and. Which Of The Following Metals Is The Best Reducing Agent.
From www.youtube.com
Which Reducing Agent is the Best? YouTube Which Of The Following Metals Is The Best Reducing Agent All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly easily. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). The strongest reducing agents. Which Of The Following Metals Is The Best Reducing Agent.
From www.slideserve.com
PPT Oxidizing & Reducing Agents PowerPoint Presentation, free Which Of The Following Metals Is The Best Reducing Agent All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly easily. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. Which of the following metal cations is the best. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. Which of the following. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved 16. Which of the following is the best reducing Which Of The Following Metals Is The Best Reducing Agent The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. The standard reduction potentials can be. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Which metal is the best reducing agent? Select the Which Of The Following Metals Is The Best Reducing Agent The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. A reducing agent is oxidized, because it loses electrons in the redox reaction. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. The standard reduction potentials can be. A reducing agent is typically in one of. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved ment 3 pter 18 Question 110 Part A Which of the Which Of The Following Metals Is The Best Reducing Agent A reducing agent loses electrons and is oxidized in a chemical reaction. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. Which of the following metals is the best reducing agent? All metals have low ionization. Which Of The Following Metals Is The Best Reducing Agent.
From www.numerade.com
SOLVED Which one of the below species will be the strongest reducing Which Of The Following Metals Is The Best Reducing Agent All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly easily. A reducing agent loses electrons and is oxidized in a chemical reaction. A reducing agent is oxidized, because it loses electrons in the redox reaction. A) ni b) mg c) fe d) co 2. Barium releases a lot of energy when oxidized to. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Part A Which of the following metals is the best Which Of The Following Metals Is The Best Reducing Agent A reducing agent is oxidized, because it loses electrons in the redox reaction. The standard reduction potentials can be. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. A reducing agent loses electrons and is oxidized in a chemical reaction. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Which the following is the best reducing agent? Cl2 Which Of The Following Metals Is The Best Reducing Agent A) ni b) mg c) fe d) co 2. Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). Which of the following metal cations is the best. Which of the following metals is the best reducing agent? All metals have low ionization energies and are relatively electropositive,. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Which of the following metals is the best reducing Which Of The Following Metals Is The Best Reducing Agent A) ni b) mg c) fe d) co 2. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Barium releases a lot of energy when oxidized to bax2+ b a x 2. Which Of The Following Metals Is The Best Reducing Agent.
From www.slideserve.com
PPT Chapter 18 Electrochemistry PowerPoint Presentation, free Which Of The Following Metals Is The Best Reducing Agent A reducing agent is oxidized, because it loses electrons in the redox reaction. The standard reduction potentials can be. All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly easily. The correct answer for reducing agent is barium (ba), as can be read from the table you provided. A reducing agent is typically in. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Which of the following is the best reducing agent? Which Of The Following Metals Is The Best Reducing Agent The correct answer for reducing agent is barium (ba), as can be read from the table you provided. The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. The standard reduction potentials can be. All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly. Which Of The Following Metals Is The Best Reducing Agent.
From chemistrytalk.org
Chemtalk Infographics ChemTalk Which Of The Following Metals Is The Best Reducing Agent A reducing agent is oxidized, because it loses electrons in the redox reaction. Which of the following metal cations is the best. A) ni b) mg c) fe d) co 2. A reducing agent loses electrons and is oxidized in a chemical reaction. All metals have low ionization energies and are relatively electropositive, and so they lose electrons fairly easily.. Which Of The Following Metals Is The Best Reducing Agent.
From glenn-has-sanford.blogspot.com
How to Tell Which Element Is Best Reducing Agent GlennhasSanford Which Of The Following Metals Is The Best Reducing Agent A reducing agent loses electrons and is oxidized in a chemical reaction. A reducing agent is oxidized, because it loses electrons in the redox reaction. A) ni b) mg c) fe d) co 2. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. All metals have low ionization energies and are relatively. Which Of The Following Metals Is The Best Reducing Agent.
From abram-blogphelps.blogspot.com
What is a Reducing Agent Which Of The Following Metals Is The Best Reducing Agent The standard reduction potentials can be. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. Which of the following metals is the best reducing agent? The correct answer for reducing agent is. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Which metal is the best reducing agent? &° (V) Which Of The Following Metals Is The Best Reducing Agent The correct answer for reducing agent is barium (ba), as can be read from the table you provided. Which of the following metals is the best reducing agent? A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. All metals have low ionization energies and are relatively electropositive, and so. Which Of The Following Metals Is The Best Reducing Agent.
From www.docsity.com
Organic chemistry reducing agents Schemes and Mind Maps Organic Which Of The Following Metals Is The Best Reducing Agent A reducing agent is oxidized, because it loses electrons in the redox reaction. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Which of the following metals is the best reducing agent?. Which Of The Following Metals Is The Best Reducing Agent.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3527121 Which Of The Following Metals Is The Best Reducing Agent Which of the following metals is the best reducing agent? The standard reduction potentials can be. A reducing agent loses electrons and is oxidized in a chemical reaction. Which of the following metal cations is the best. Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. The strongest reducing agents are the alkali metals (group 1). Which Of The Following Metals Is The Best Reducing Agent.
From www.numerade.com
SOLVEDArrange the following metals in order of increasing strength as Which Of The Following Metals Is The Best Reducing Agent A reducing agent is typically in one of its lower possible oxidation states, and is known as the electron donor. Lithium metal is therefore the strongest reductant (most easily oxidized) of the alkali metals in aqueous solution. The standard reduction potentials can be. The correct answer for reducing agent is barium (ba), as can be read from the table you. Which Of The Following Metals Is The Best Reducing Agent.
From www.doubtnut.com
The metal which is best reducing agent/has lowest reduction potential Which Of The Following Metals Is The Best Reducing Agent Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. The strongest reducing agents are the alkali metals (group 1) as they have low electronegativities and lose electrons very easily. The correct answer for reducing agent is barium (ba), as can be read from the table you provided. Barium releases a lot of energy when oxidized to. Which Of The Following Metals Is The Best Reducing Agent.
From general.chemistrysteps.com
Oxidizing and Reducing Agents Chemistry Steps Which Of The Following Metals Is The Best Reducing Agent Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). Which of the following metals is the best reducing agent? The correct answer for reducing agent is barium (ba), as can be read from the table you provided. Which of the following metal cations is the best. Lithium. Which Of The Following Metals Is The Best Reducing Agent.
From www.chegg.com
Solved Which of the following metals is the best reducing Which Of The Following Metals Is The Best Reducing Agent Examples of reducing agents include the earth metals, formic acid, and sulfite compounds. Which of the following metals is the best reducing agent? Barium releases a lot of energy when oxidized to bax2+ b a x 2 + (2.9 ev 2.9 e v per elevtron). The correct answer for reducing agent is barium (ba), as can be read from the. Which Of The Following Metals Is The Best Reducing Agent.