Fda Guidelines For Sterilization Of Medical Devices . this guidance discusses the information to be submitted by device manufacturers regarding medical device. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic.
from mavink.com
submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. this guidance discusses the information to be submitted by device manufacturers regarding medical device. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices.
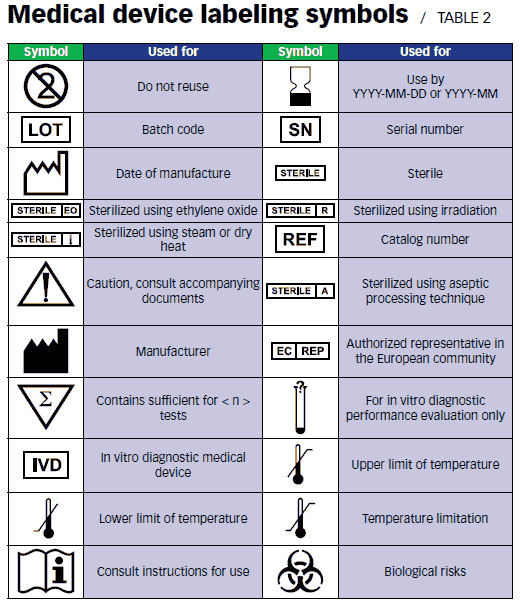
Medical Device Labeling Symbols
Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. this guidance discusses the information to be submitted by device manufacturers regarding medical device. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable.
From www.scribd.com
FDA Requirements for Medical Devices Medical Device Authentication Fda Guidelines For Sterilization Of Medical Devices this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this guidance discusses the information to be submitted by device. Fda Guidelines For Sterilization Of Medical Devices.
From www.alamy.com
Full vector set of sterilized and disinfectant symbols for medical Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. submission and review of sterility information in premarket notification (510(k)) submissions. Fda Guidelines For Sterilization Of Medical Devices.
From fdareporter.com
U.S. FDA Statement on new steps to advance innovation in medical Fda Guidelines For Sterilization Of Medical Devices submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this guidance replaces the 1987 industry guideline on sterile drug products produced by. Fda Guidelines For Sterilization Of Medical Devices.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Fda Guidelines For Sterilization Of Medical Devices learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this guidance discusses the information to be submitted by device. Fda Guidelines For Sterilization Of Medical Devices.
From dimensionsofdentalhygiene.com
Ensure Infection Control Compliance With Chemical and Biological Fda Guidelines For Sterilization Of Medical Devices this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this guidance discusses the information to be submitted by device manufacturers. Fda Guidelines For Sterilization Of Medical Devices.
From www.regdesk.co
FDA Guidance on Surgical Staplers and Staples Technical Fda Guidelines For Sterilization Of Medical Devices learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. this guidance discusses the information to be submitted by device manufacturers regarding medical device. . Fda Guidelines For Sterilization Of Medical Devices.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Fda Guidelines For Sterilization Of Medical Devices learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. this document provides guidance for industry and fda staff on how. Fda Guidelines For Sterilization Of Medical Devices.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this document provides nonbinding recommendations for current good manufacturing practice (cgmp). Fda Guidelines For Sterilization Of Medical Devices.
From amactechnologies.com
Sterile Medical Device Packaging Automated Vacuum Sealer Fda Guidelines For Sterilization Of Medical Devices this guidance discusses the information to be submitted by device manufacturers regarding medical device. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. learn how fda inspects the validation, monitoring and control. Fda Guidelines For Sterilization Of Medical Devices.
From www.youtube.com
Guidance for Cleaning, Disinfection and Sterilization of Reusable Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. this guidance discusses the information to be submitted by device manufacturers regarding medical device. this document provides guidance for industry and fda staff on how to validate. Fda Guidelines For Sterilization Of Medical Devices.
From dxjapjmqeco.blob.core.windows.net
Sterilization Of Health Care Products Radiation at Donald Lineberger blog Fda Guidelines For Sterilization Of Medical Devices submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance replaces the 1987 industry guideline on sterile drug products produced. Fda Guidelines For Sterilization Of Medical Devices.
From www.medpagetoday.com
FDA Panel Urges New Label, More Guidance on Female Sterilization Device Fda Guidelines For Sterilization Of Medical Devices this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance discusses the information to be submitted by device manufacturers regarding medical device. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. learn how fda inspects the validation, monitoring and control of sterilization. Fda Guidelines For Sterilization Of Medical Devices.
From www.modernhealthcare.com
Why is carcinogen ethylene oxide used to sterilize medical devices Fda Guidelines For Sterilization Of Medical Devices submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this document provides nonbinding recommendations for current good manufacturing. Fda Guidelines For Sterilization Of Medical Devices.
From medicom-asia.com
Sterilization Products Asia Fda Guidelines For Sterilization Of Medical Devices this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. this guidance discusses the information to be submitted by device manufacturers regarding medical device. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. . Fda Guidelines For Sterilization Of Medical Devices.
From microbeonline.com
Sterilization and Disinfection Methods • Microbe Online Fda Guidelines For Sterilization Of Medical Devices this guidance discusses the information to be submitted by device manufacturers regarding medical device. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. learn how fda inspects the validation, monitoring and control. Fda Guidelines For Sterilization Of Medical Devices.
From operonstrategist.com
FDA's Updated Sterilization Guidelines for Medical Device Operon Fda Guidelines For Sterilization Of Medical Devices this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this document provides nonbinding recommendations for current good. Fda Guidelines For Sterilization Of Medical Devices.
From kerone.com
Different Types of Sterilization Process Fda Guidelines For Sterilization Of Medical Devices submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. learn how fda inspects the validation, monitoring and control of sterilization. Fda Guidelines For Sterilization Of Medical Devices.
From www.researchgate.net
(PDF) APSIC guidelines for disinfection and sterilization of Fda Guidelines For Sterilization Of Medical Devices this guidance discusses the information to be submitted by device manufacturers regarding medical device. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. learn how fda inspects the validation, monitoring and control of sterilization processes. Fda Guidelines For Sterilization Of Medical Devices.
From www.eetimes.com
Sterilization methods and impact on electronics in medical devices EE Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this document provides nonbinding recommendations for current good manufacturing. Fda Guidelines For Sterilization Of Medical Devices.
From www.raps.org
FDA recognizes new medical device sterilization standards RAPS Fda Guidelines For Sterilization Of Medical Devices this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. learn how fda inspects the validation, monitoring and control. Fda Guidelines For Sterilization Of Medical Devices.
From medicaldeviceacademy.com
How to select and help validate the best sterilization method? Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. learn about the different methods and standards for sterilizing. Fda Guidelines For Sterilization Of Medical Devices.
From www.researchgate.net
(PDF) Evaluation of Sterilization Methods for Medical Devices Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. this guidance discusses the information to be submitted by device manufacturers regarding medical device. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. submission and review of sterility information in premarket notification (510(k)) submissions for devices. Fda Guidelines For Sterilization Of Medical Devices.
From cmo.fresenius-kabi.com
Our Sterile Pharmaceutical Technologies Fresenius Kabi Contract Fda Guidelines For Sterilization Of Medical Devices learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance discusses the information to be submitted by device. Fda Guidelines For Sterilization Of Medical Devices.
From cecvktal.blob.core.windows.net
What Is The Best Method For Determining The Quality Of Sterilization at Fda Guidelines For Sterilization Of Medical Devices learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. this guidance discusses the information to be submitted by device manufacturers regarding medical device. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance replaces the 1987 industry guideline on sterile drug products. Fda Guidelines For Sterilization Of Medical Devices.
From www.steris-ast.com
Comparison of AAMI Methods for Setting of Minimum Sterilization Dose Fda Guidelines For Sterilization Of Medical Devices this guidance discusses the information to be submitted by device manufacturers regarding medical device. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. learn how fda inspects the validation, monitoring and control. Fda Guidelines For Sterilization Of Medical Devices.
From ceccoyut.blob.core.windows.net
Fda Guidance Shelf Life Of Medical Devices at Kevin Cloutier blog Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this guidance discusses the information to be submitted by device manufacturers regarding medical device. this document provides nonbinding recommendations for current good manufacturing practice (cgmp). Fda Guidelines For Sterilization Of Medical Devices.
From www.qualitymeddev.com
Gamma Sterilization Process for Medical Devices QualityMedDev Fda Guidelines For Sterilization Of Medical Devices this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. this guidance replaces the 1987 industry guideline on sterile drug products produced. Fda Guidelines For Sterilization Of Medical Devices.
From angelanjohnson.com
Medical Devices Angela N Johnson Fda Guidelines For Sterilization Of Medical Devices this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance discusses the information to be submitted by device manufacturers regarding medical device. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this document provides nonbinding recommendations for current good manufacturing practice (cgmp). Fda Guidelines For Sterilization Of Medical Devices.
From princesterilization.com
Terminal Sterilization of Sterile Filtered Products Prince Fda Guidelines For Sterilization Of Medical Devices this guidance discusses the information to be submitted by device manufacturers regarding medical device. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. . Fda Guidelines For Sterilization Of Medical Devices.
From www.aplyon.com
Ethylene Oxide EO Sterilization Validation Procedure Fda Guidelines For Sterilization Of Medical Devices submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. this guidance replaces the 1987 industry guideline on sterile drug. Fda Guidelines For Sterilization Of Medical Devices.
From www.alamy.com
Five medical symbols about the method of sterilization on medical Fda Guidelines For Sterilization Of Medical Devices this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance discusses the information to be submitted by device manufacturers regarding medical device. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. learn about the different methods and standards for sterilizing medical. Fda Guidelines For Sterilization Of Medical Devices.
From mavink.com
Mdr Classification Chart Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. this document provides nonbinding recommendations for current good manufacturing. Fda Guidelines For Sterilization Of Medical Devices.
From cewzjuea.blob.core.windows.net
Autoclave Sterilization Of Surgical Instruments at Francisco Bryan blog Fda Guidelines For Sterilization Of Medical Devices this guidance replaces the 1987 industry guideline on sterile drug products produced by aseptic processing (aseptic. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled. learn how fda inspects the validation, monitoring and control of sterilization processes. Fda Guidelines For Sterilization Of Medical Devices.
From mavink.com
Medical Device Labeling Symbols Fda Guidelines For Sterilization Of Medical Devices this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this guidance discusses the information to be submitted by device manufacturers regarding medical device. learn about the different methods and standards for sterilizing medical devices, including ethylene oxide,. submission and review of sterility information in premarket notification (510(k)) submissions. Fda Guidelines For Sterilization Of Medical Devices.
From remmed.com
FDA Registered Medical Devices What to Expect Remington Fda Guidelines For Sterilization Of Medical Devices this document provides guidance for industry and fda staff on how to validate reprocessing methods for reusable. this document provides nonbinding recommendations for current good manufacturing practice (cgmp) of sterile. learn how fda inspects the validation, monitoring and control of sterilization processes for medical devices. learn about the different methods and standards for sterilizing medical devices,. Fda Guidelines For Sterilization Of Medical Devices.